Significantly clearer skin
SKIN CLEARANCE AT WEEKS 16 AND 52
(primary and secondary endpoints,
respectively)1,2,a-e
Definitive conclusions cannot be made at Week 4, as this was a post hoc analysis. Data were not multiplicity controlled and P value was nominal.
39%
of DUPIXENT + TCS adult patients achieved clear or almost-clear skin at Week 16
vs 12% with placebo + TCS (primary endpoint; P<0.0001)2,3
Significant skin clearance was also demonstrated with DUPIXENT in monotherapy trials3,4
38%
of adults treated with DUPIXENT (n=224) achieved clear or almost-clear skin
(primary endpoint) vs 10% with placebo at Week 16 in SOLO 1 (n=224; P<0.001)
36%
of adults treated with DUPIXENT (n=233) achieved clear or almost-clear skin
(primary endpoint) vs 9% with placebo at Week 16 in SOLO 2 (n=236; P<0.001)
Patient 1: achieved a 3‑point improvement in IGA
RESULTS
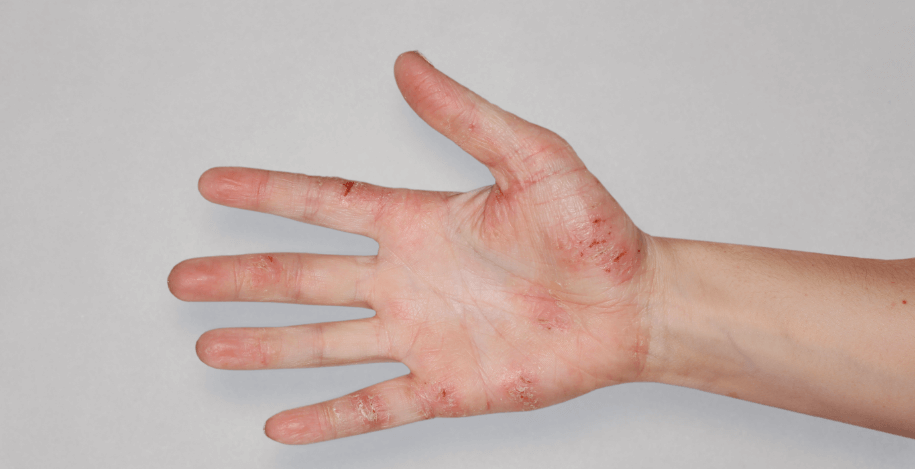
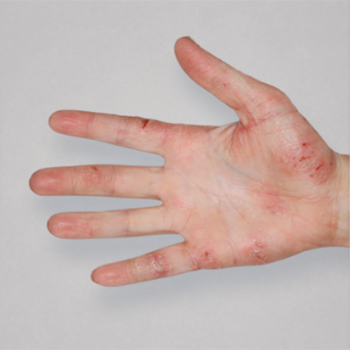
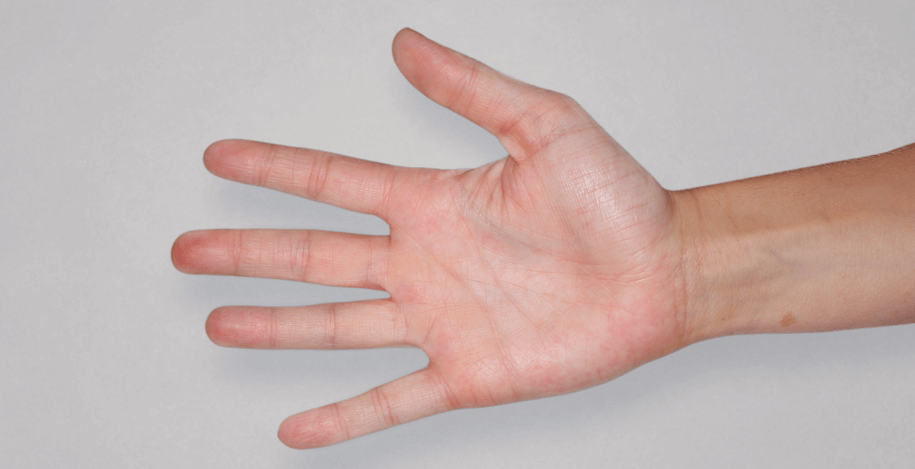
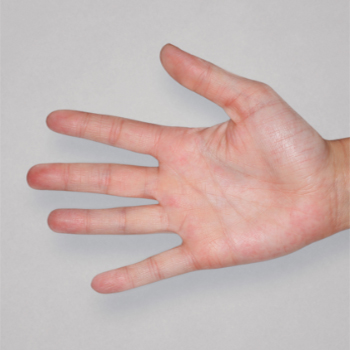
Patient 2: achieved a 3‑point improvement in IGA
RESULTS
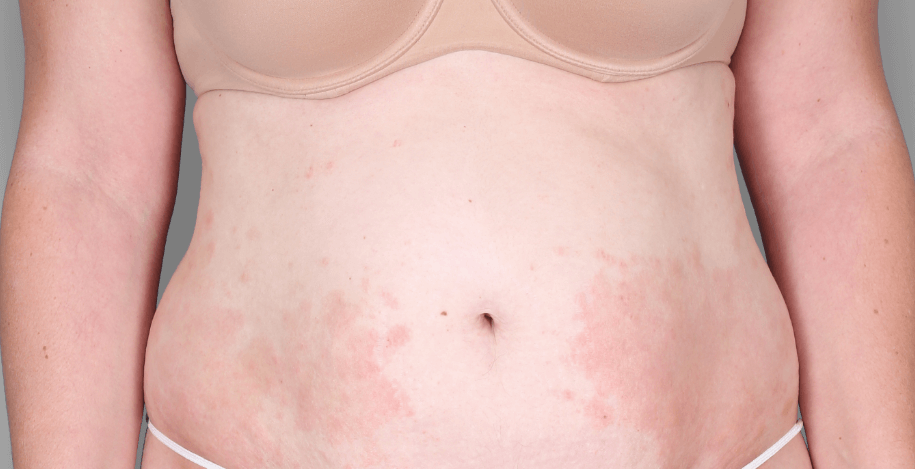
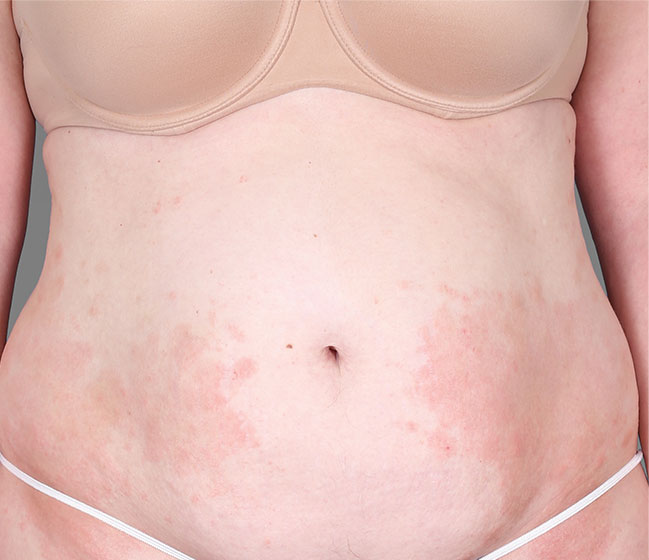
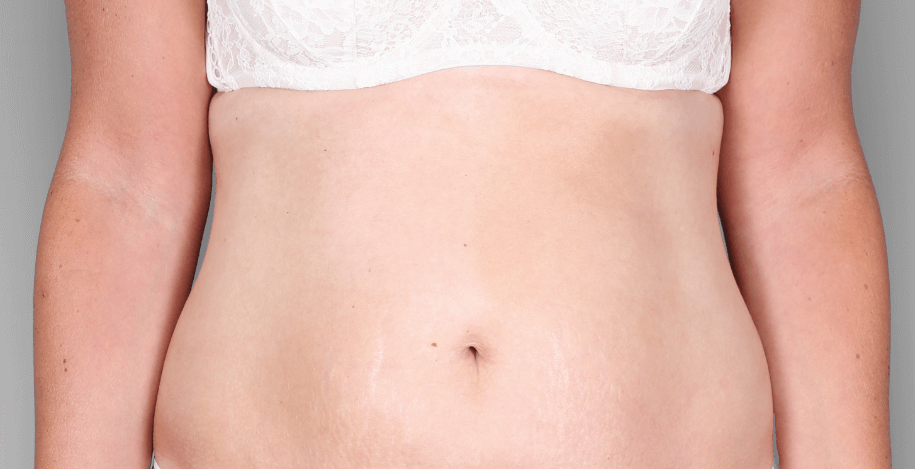
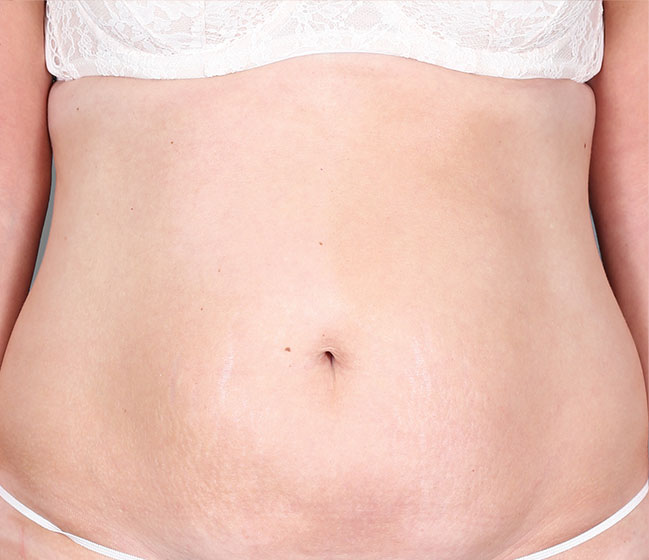
Patient 3: achieved a 4‑point improvement in IGA
RESULTS
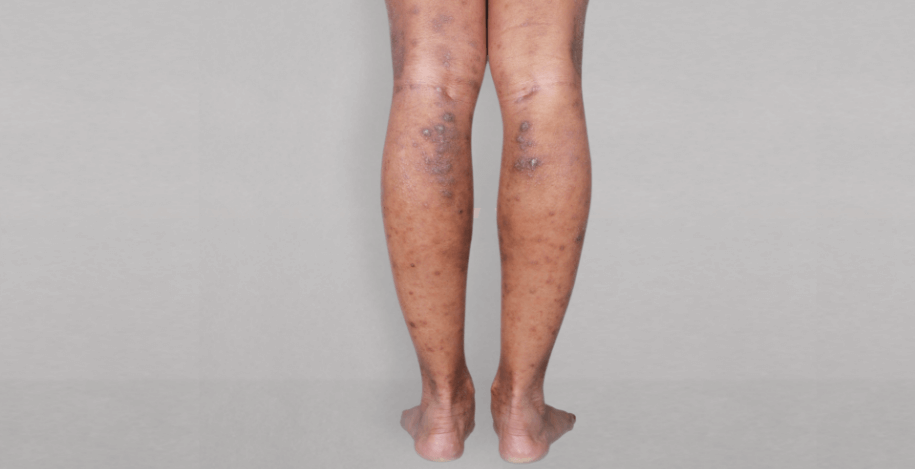
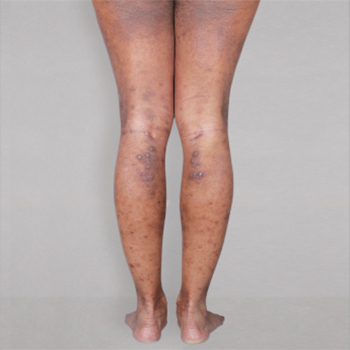
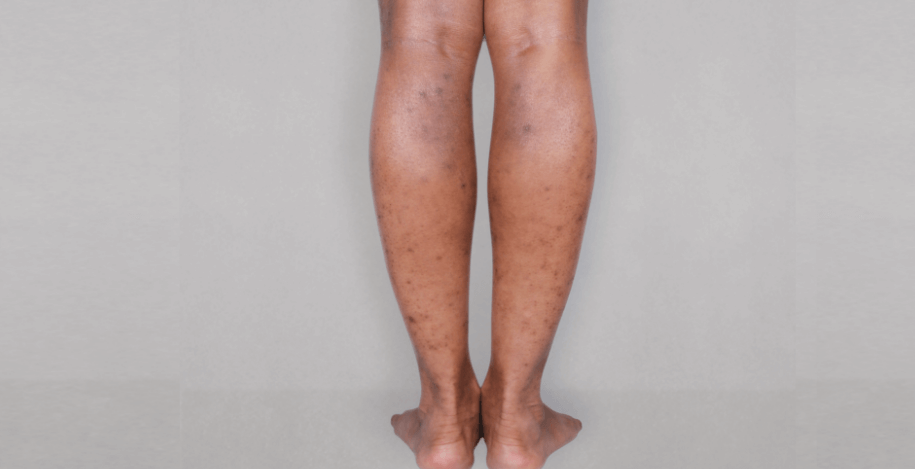
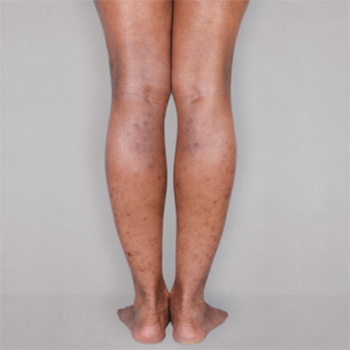
Visible results seen in real-world patients
In DUPIXENT clinical trials including adults, the primary endpoint was the change from baseline in the proportion of subjects with an Investigator’s Global Assessment (IGA) of 0 (clear) or 1 (almost clear) and ≥2-point improvement at Week 16.3
Actual patients treated with DUPIXENT. Not clinical trial patients. Patients 2 and 3 were on concomitant therapies, such as TCS, phototherapy, etc, at their prescribing healthcare professional’s discretion. Scoring was designated by the treating healthcare professional. Because all 3 patients were real-world patients, there may be other factors influencing their treatment results, and individual results may vary.
aA clinical responder was defined as a subject with IGA 0 or 1 (clear or almost clear) with a reduction of ≥2 points from baseline on a 0-4 IGA scale at Week 16 in all
trials (primary efficacy outcome) and at Week 52 in CHRONOS (other endpoint).3
bIn the primary analyses of the efficacy endpoints, subjects who received rescue treatment or with missing data were considered nonresponders.3
cFull Analysis Set includes all subjects randomized.3
dIn CHRONOS, as needed, subjects received topical calcineurin inhibitors for problem areas only, such as the face, neck, and intertriginous and genital areas.3
eWeek 52 data were limited to patients completing 52 weeks as of the cutoff date.3
IGA assesses the overall severity of the clinical signs of atopic dermatitis1
A 0- to 4-point scoring system of the overall severity of atopic dermatitis skin lesions2
Severe
Disease
Severe erythema and severe
papulation/infiltration
Moderate
Disease
Moderate erythema and moderate
papulation/infiltration
Mild
Disease
Mild erythema and mild
papulation/infiltration
Almost
Clear
Just perceptible erythema, and
just perceptible papulation/infiltration
Clear
No inflammatory signs of atopic
dermatitis
A clinical responder
was defined as a
patient achieving
IGA 0 or 1 and at least
a 2-point improvement
from baseline1
DATA FROM REAL‑WORLD EXPERIENCE