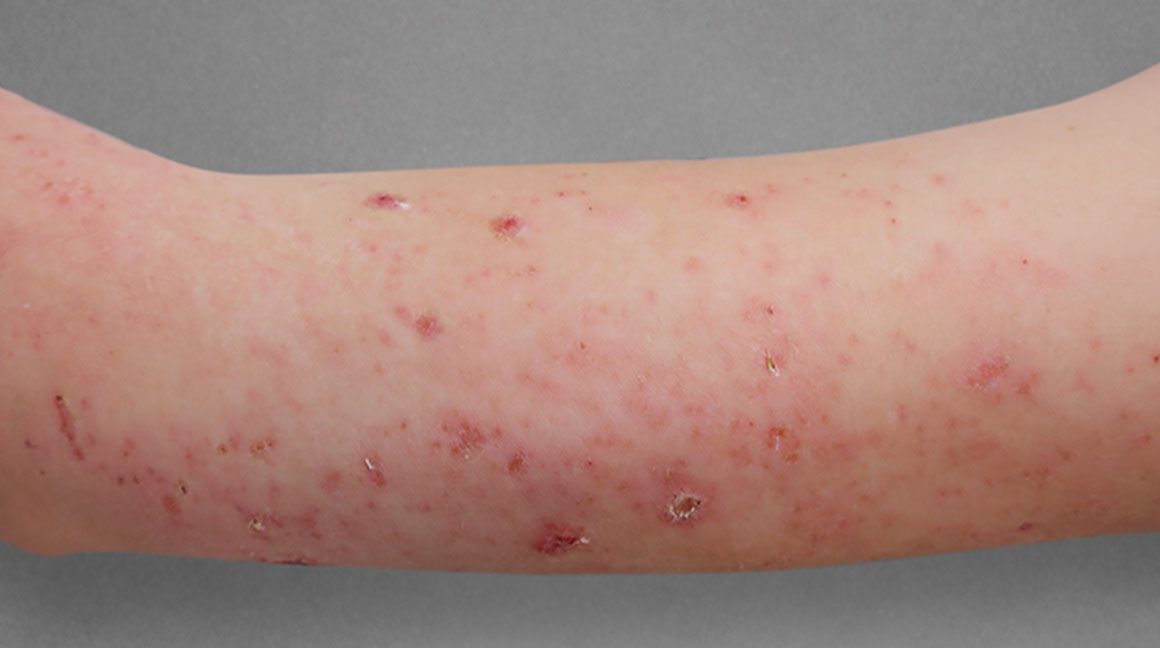
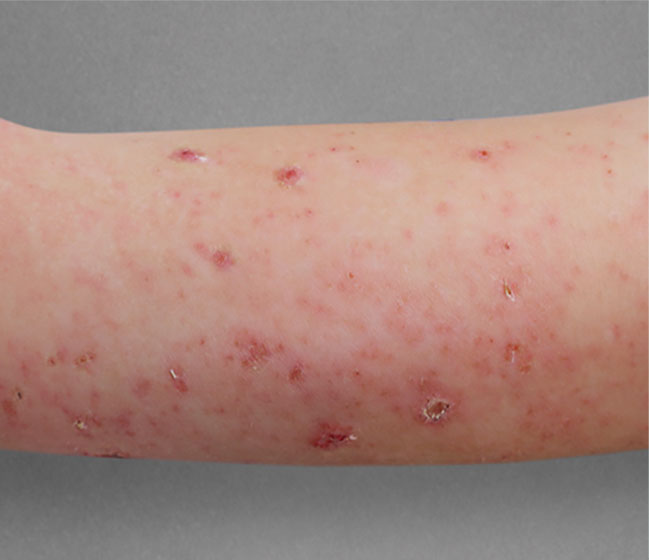
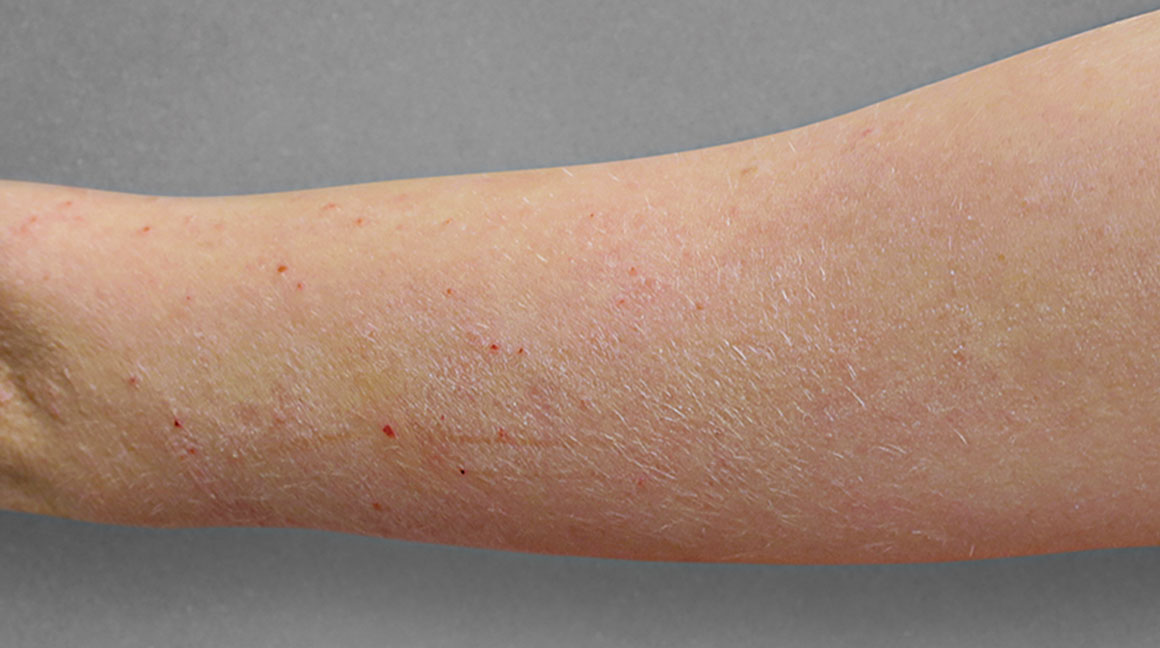
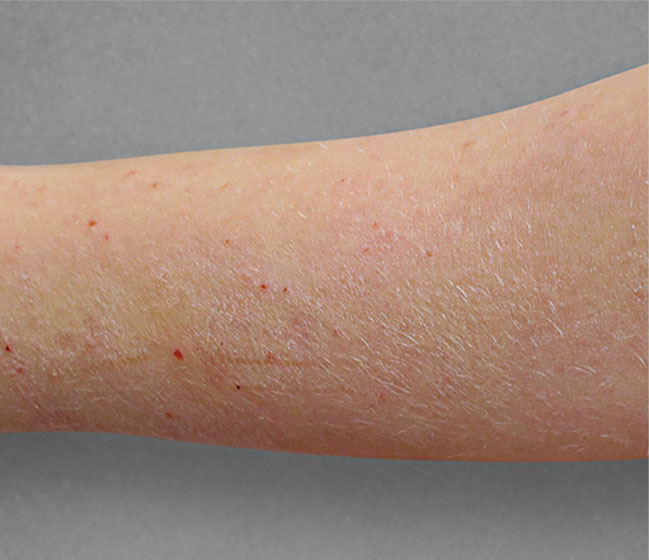
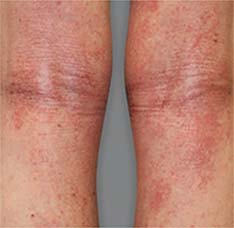
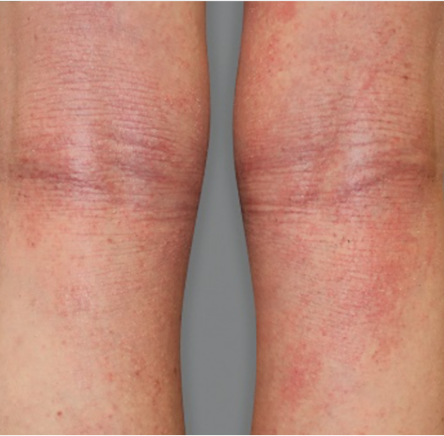
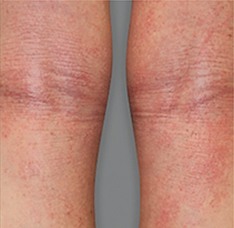
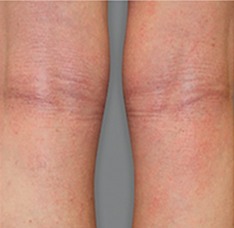
Significant skin clearance (primary endpoint; P<0.001)1,2,a
|
DUPIXENT (n=82)c |
PLACEBO (n=85) |
|
|---|---|---|
|
Achieved clear or almost‑clear skin
(IGA 0 or 1) |
24% | 2% |
aA clinical responder was defined as a subject with an IGA 0 or 1 (clear or almost clear) and a reduction of ≥2 points on a 0‑4 scale at Week 16 (primary efficacy outcome).1
bFull Analysis Set includes all subjects randomized.1
cAdolescents ≥60 kg received DUPIXENT 300 mg Q2W after a 600 mg loading dose, and adolescents <60 kg received 200 mg Q2W after a 400 mg loading dose.1