Skin clearance demonstrated in a phase 3 study of patients 12+ years of age1-3
Consider the BURDEN
that
SMALL BSA
involvement
can have4-6
Not an actual patient.
IGA hand and foot efficacy results
Not an actual patient.
As many patients
taking DUPIXENT achieved skin clearance at Week 16 vs placebo1-3
taking DUPIXENT achieved skin
clearance at Week 16 vs placebo1-3
BSA, body surface area; IGA, Investigator’s Global Assessment.
All DUPIXENT-treated patients in AD-HAFT received DUPIXENT monotherapy for their hand and/or foot lesions3
- Dosing for DUPIXENT in this study was consistent with the approved dosage in the Prescribing Information1,3
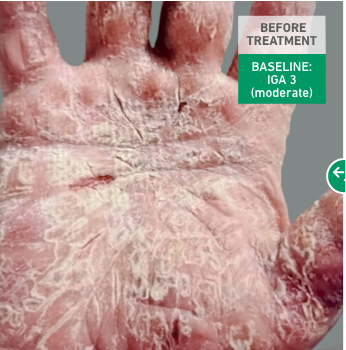
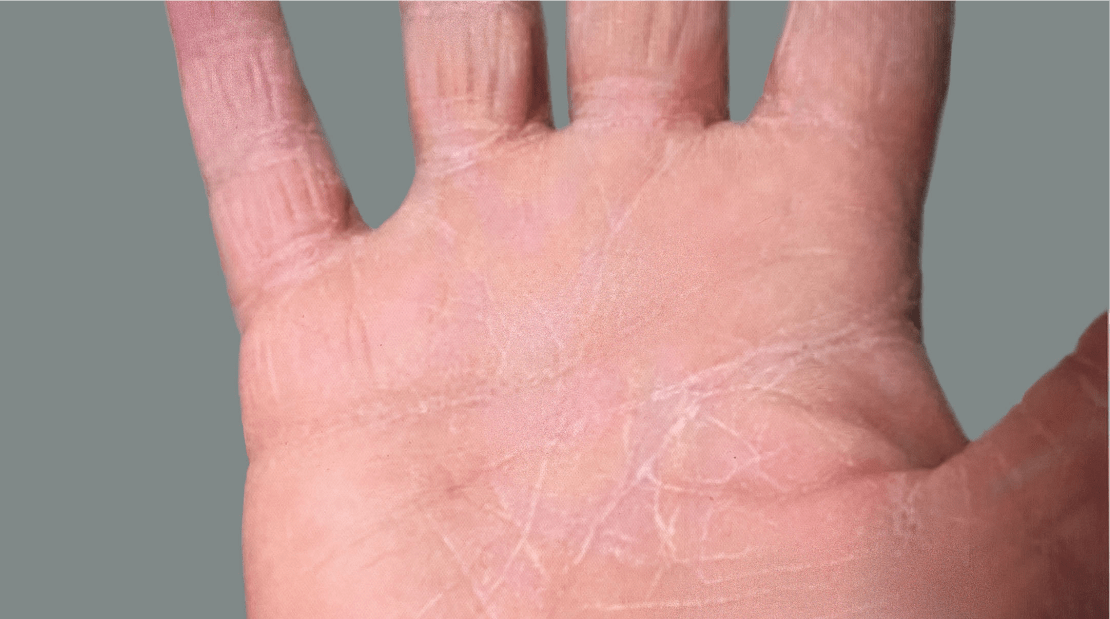
Adult patient 1: Achieved a 3-point improvement in IGA hand and foot score
This adult patient was an actual patient (not a clinical trial patient) treated with DUPIXENT. Scoring was designated by the treating healthcare professional. Because this was a real-world patient, other factors, including concomitant therapies, may have influenced their treatment results, and individual results may vary.
TO SEE
RESULTS

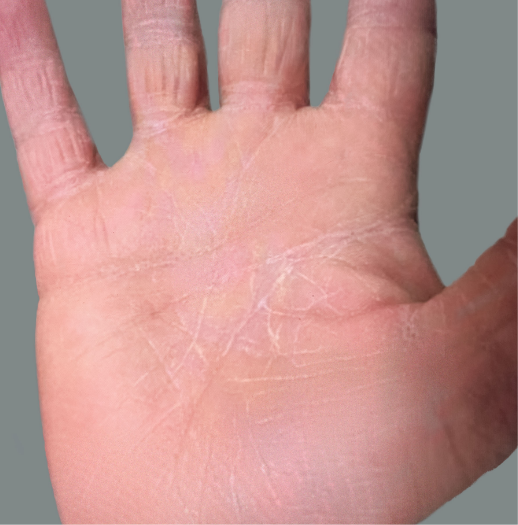
Adult patient 2: Achieved a 3-point improvement in IGA hand and foot score
This adult patient was an actual patient (not a clinical trial patient) treated with DUPIXENT. Scoring was designated by the treating healthcare professional. Because this was a real-world patient, other factors, including concomitant therapies, may have influenced their treatment results, and individual results may vary.
TO SEE
RESULTS
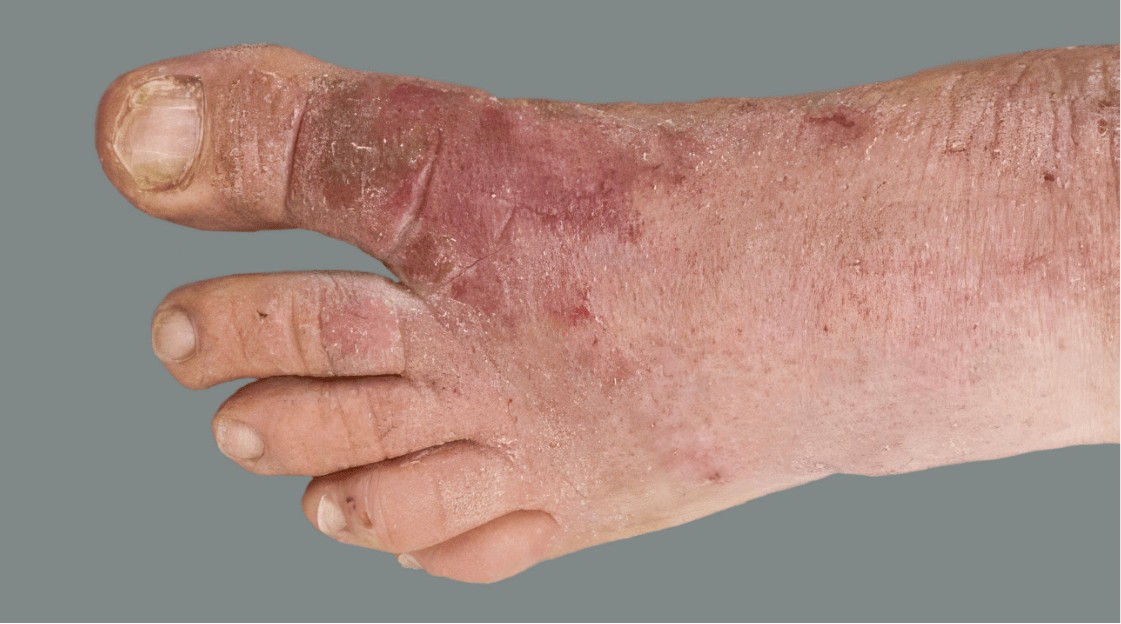
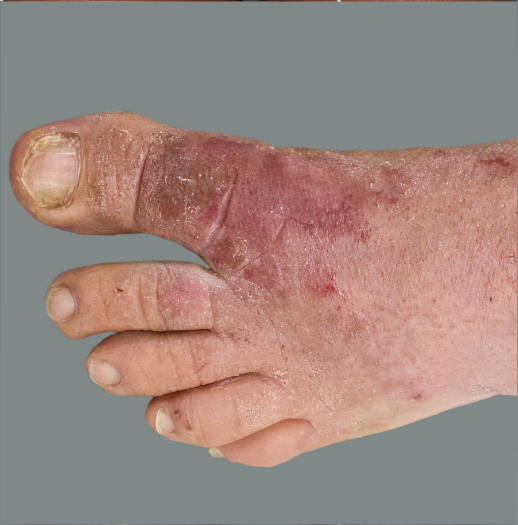
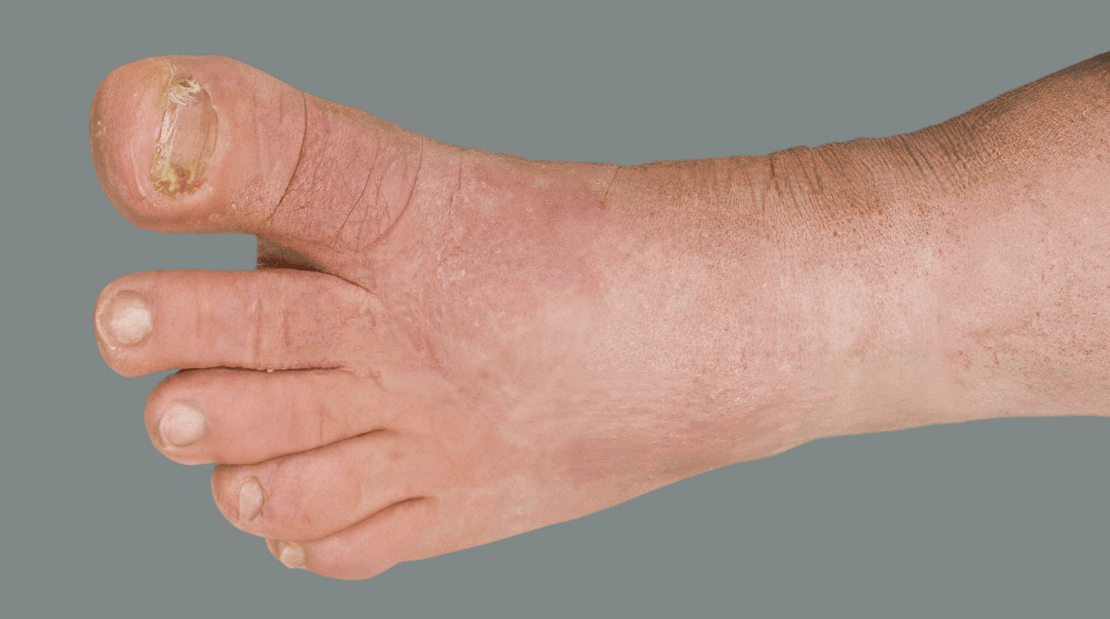
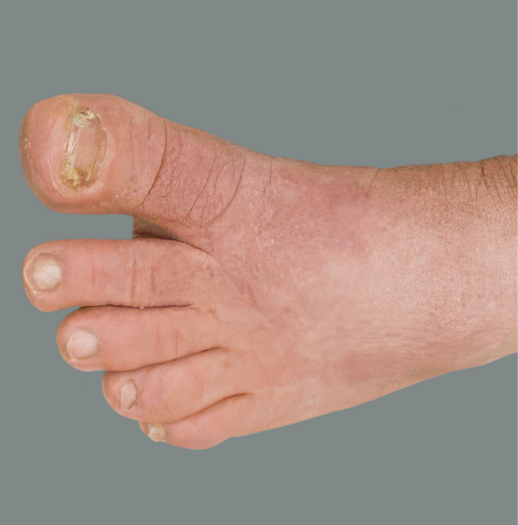
A clinical responder in the clinical trial of patients with uncontrolled moderate-to-severe atopic hand and foot dermatitis was defined as a patient achieving IGA hand and foot 0 or 1.1
Explore more IGA efficacy results
(12 TO 17 YEARS) DATA VIEW CHILD (6 TO 11 YEARS) DATA VIEW INFANT TO PRESCHOOLER
(6 MONTHS TO 5 YEARS) DATA
BSA, body surface area; IGA, Investigator’s Global Assessment; Q2W, once every 2 weeks.
ready TO prescribe dupixent?