DUPIXENT does not require initial lab testing or ongoing lab monitoring,
according to the Prescribing Information1
One regimen
18+ YEARS
One dosage
regimen
in adults,
regardless
of
weight
Initial loading dose:
600 mg
2 x 300 mg
Followed by:
300 mg Q2W
1 x 300 mg
Weight-tiered
6 TO 17 YEARSa
≥60 kg
Initial loading dose:
600 mg
2 x 300 mg
Followed by:
300 mg Q2W
1 x 300 mg
30 to <60 kg
Initial loading dose:
400 mg
2 x 200 mg
Followed by:
200 mg Q2W
1 x 200 mg
15 to <30 kg
Initial loading dose:
600 mg
2 x 300 mg
Followed by:
300 mg Q4W
1 x 300 mg
6 MONTHS
TO 5 YEARSa
15 to <30 kg
No initial loading dose
recommended
Initial and subsequent dosage
300 mg Q4W
1 x 300 mg
5 to <15 kg
No initial loading dose
recommended
Initial and subsequent dosage
200 mg Q4W
1 x 200 mg
Get the full
dosing regimen
Email Dosing Regimen
PRINT DOSING REGIMEN
For supplemental injection training, visit our Patient Video Injection Support page
AD, atopic dermatitis; Q2W, once every 2 weeks; Q4W, once every 4 weeks.
a5 kg is equal to 11 lb; 15 kg is equal to 33 lb;
30 kg is equal to 66 lb; 60 kg is equal to 132 lb.
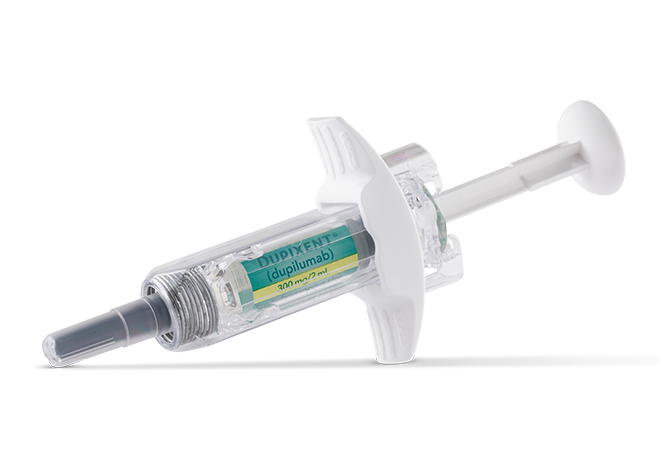
Available in a 200 mg and 300 mg pre-filled pen (for indicated patients 2+ years of age) or pre-filled syringe (for indicated patients 6+ months of age) for subcutaneous injection1,2
At-home or in-office administration options
Consider before injecting
- DUPIXENT is an injectable medicine that is administered by subcutaneous injection and is intended for use under the guidance of a healthcare provider1
- A patient may self-inject DUPIXENT—or a caregiver may administer DUPIXENT—after training has been provided by a healthcare provider on proper subcutaneous injection technique using the pre-filled syringe or pre-filled pen1
- In children 6 months to less than 12 years of age, DUPIXENT should be given by a caregiver
- In children 12 years of age and older, it is recommended that DUPIXENT be administered by or under the
supervision of an adult
- It is important to provide proper training to patients and/or caregivers on the preparation and administration of DUPIXENT prior to use1
- After administration, advise patients to follow sharps disposal recommendations1
- Patients and/or caregivers should read all the Instructions for Use—either 200 mg or 300 mg pre-filled pen or pre-filled syringe—prior to injecting1
- Consider completing all age-appropriate vaccinations as recommended by current immunization guidelines prior to initiating treatment with DUPIXENT1
Important administration
instructions1,3
Preparation and administration of DUPIXENT
- Patients and/or caregivers should read the Instructions for Use prior to injecting
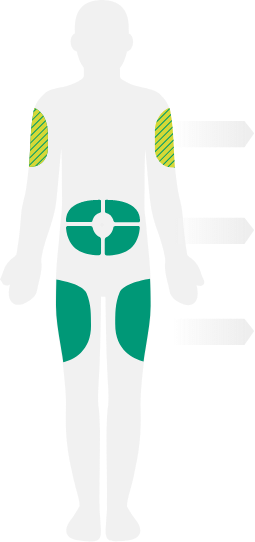
- Instruct patients and/or caregivers to administer the subcutaneous injection into the thigh or abdomen,
except for the 2 inches (5 cm) around the navel - The upper arm can also be used if a caregiver administers the injection
- It is important to rotate the injection site with each injection. DO NOT inject DUPIXENT
into skin that is tender, damaged, bruised, or scarred - For the initial dose, patients and/or caregivers should administer each DUPIXENT
injection at a different injection site - In children 6 months to less than 12 years of age, DUPIXENT should be given by a caregiver.
In children 12 years of age and older, it is recommended that DUPIXENT be administered
by or under the supervision of an adult
See the Instructions for Use for more detailed instructions on the preparation and administration of DUPIXENT
For supplemental injection training, visit our
Patient Video Injection Support Page
Additional dosage
information
Before injection, instruct patients and/or caregivers to remove the DUPIXENT pre-filled pen or syringe from the refrigerator and allow DUPIXENT to reach room temperature without removing the needle cap.
- 45 minutes for the 300 mg/2 mL
pre‑filled pen or syringe - 30 minutes for the 200 mg/1.14 mL
pre-filled pen or syringe
Patients and/or caregivers should not use the pre-filled pen or syringe if the liquid contains visible particulate matter, or is discolored or cloudy (other than clear to slightly opalescent, colorless to pale yellow).
Patients and/or caregivers should discard any unused product remaining in the pre-filled pen or syringe in accordance with local requirements.
For supplemental injection training, visit our Patient Video Injection Support Page
DUPIXENT is a clear to slightly opalescent, colorless to pale yellow solution available as:
- Injection: 300 mg/2 mL in a single-dose, pre-filled pen with hidden needle or syringe with needle shield
- Injection: 200 mg/1.14 mL in a single-dose, pre-filled pen with hidden needle or syringe with needle shield
DUPIXENT is available in cartons containing 2 pre-filled pens or syringes with needle shields. Each 300 mg pre-filled syringe or pre-filled pen delivers 300 mg/2 mL. Each 200 mg pre-filled syringe or pre-filled pen delivers 200 mg/1.14 mL.
- DUPIXENT is sterile and preservative-free. Patients and/or caregivers should discard any unused portion
- Patients and/or caregivers should store DUPIXENT by refrigerating between 36 °F to 46 °F (2 °C to 8 °C) in the original carton to protect from light
- If necessary, pre-filled pens or syringes may be kept at room temperature up to 77 °F (25 °C) for a maximum of 14 days. DUPIXENT should not be stored above 77 °F (25 °C). After removal from the refrigerator, DUPIXENT must be used within 14 days or discarded
- The pen or syringe should not be exposed to heat or direct sunlight
- Any unused medicinal product or waste material should be disposed of in accordance with local requirements
- Do NOT freeze. Do NOT expose to heat. Do NOT shake
DUPIXENT MyWay®