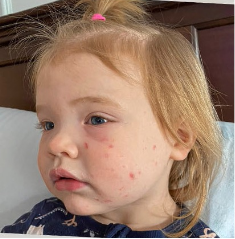
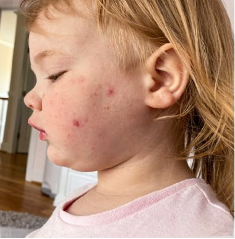
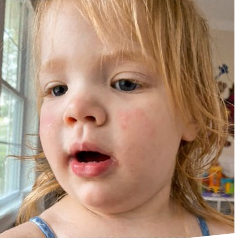
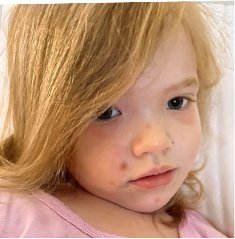
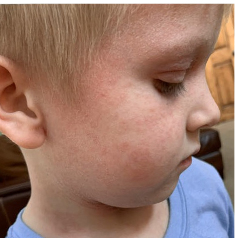
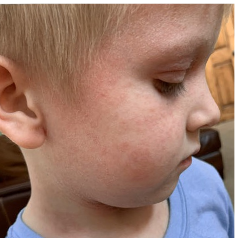
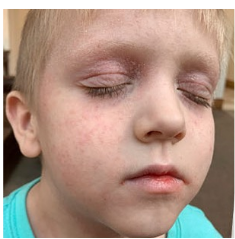
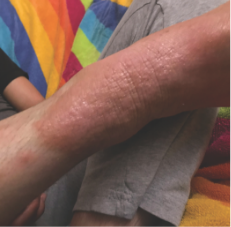
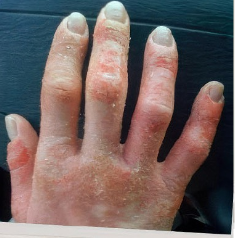
162 infants to preschoolers (6 months to 5 years) in AD-1539 (16 weeks) with moderate-to-severe atopic dermatitis inadequately controlled with topical prescription therapies were randomized to DUPIXENT + TCS or placebo + TCS. Patients 15 kg but >30 kg received 300 mg Q4W. Patients 5 kg but <15 kg received 200 mg Q4W. Patients had an IGA score ≥3 on a scale of 0 to 4, an EASI score ≥16 on a scale of 0 to 72, and BSA involvement of ≥10%. At baseline, 23% of infants to preschoolers had an IGA score of 3 (moderate), 77% had an IGA of 4 (severe), mean EASI score was 34.1, and weekly average of daily Worst Scratch/Itch NRS was 7.6 on a scale of 0 to 10.2,3
The primary endpoint was the proportion of subjects with an IGA 0 (clear) or 1 (almost clear) at Week 16 (28% of patients treated with DUPIXENT + TCS vs 4% with placebo + TCS, P<0.0001). Other endpoints included the proportion of subjects with EASI-75 at Week 16 (53% of patients treated with DUPIXENT + TCS vs 11% with placebo + TCS, P<0.0001) and ≥4-point improvement in the Worst Scratch/Itch NRS at Week 16 (48% of patients treated with DUPIXENT + TCS vs 9% with placebo + TCS, P<0.0001).2,3
The most common adverse reactions (incidence ≥1%) in patients with atopic dermatitis are injection site reactions, conjunctivitis, blepharitis, oral herpes, keratitis, eye pruritus, other herpes simplex virus infection, dry eye, and eosinophilia. The long-term safety profile of DUPIXENT ± TCS observed in infants to preschoolers was consistent with that seen in adults, adolescents and children with atopic dermatitis. In AD-1434, hand-foot-and-mouth disease and skin papilloma (incidence ≥2%) was reported in infants to preschoolers. These cases did not lead to study drug discontinuation.2
BSA, body surface area; EASI, Eczema Area and Severity Index; IGA, Investigator’s Global Assessment; NRS, numerical rating scale; Q4W, once every 4 weeks; TCS, topical corticosteroids.