Real-world visible results
These adult patients were actual patients (not clinical trial patients) treated with DUPIXENT for ≥24 weeks. Scoring was designated by the healthcare professionals. Because these patients were real-world patients, other factors may have influenced treatment results, and individual results may vary.
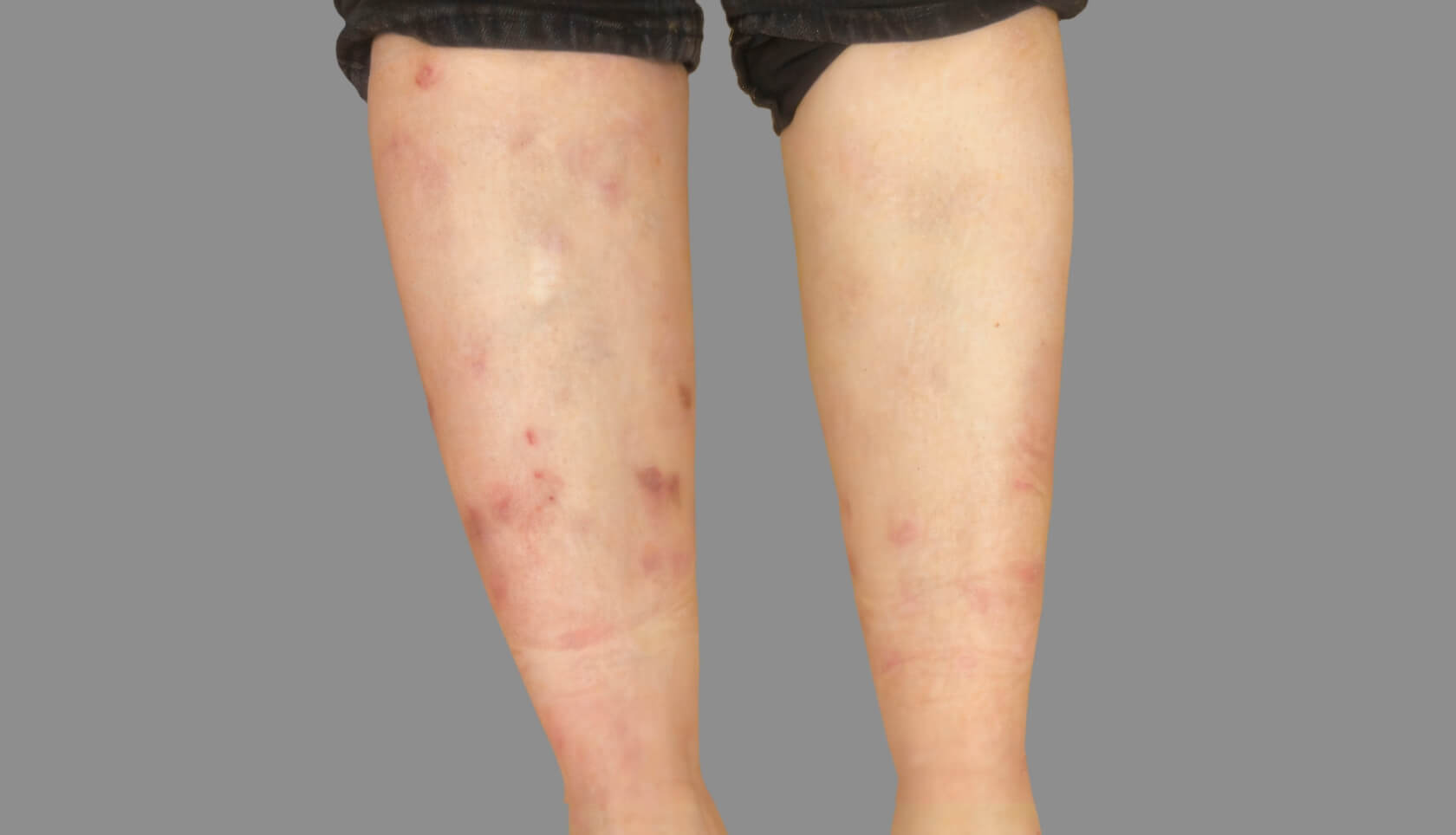
PATIENT 1: 62-YEAR-OLD FEMALE PATIENT WITH A
3-POINT IMPROVEMENT IN IGA PN-S
Current PN therapy: DUPIXENT 300 mg Q2W
RESULTS
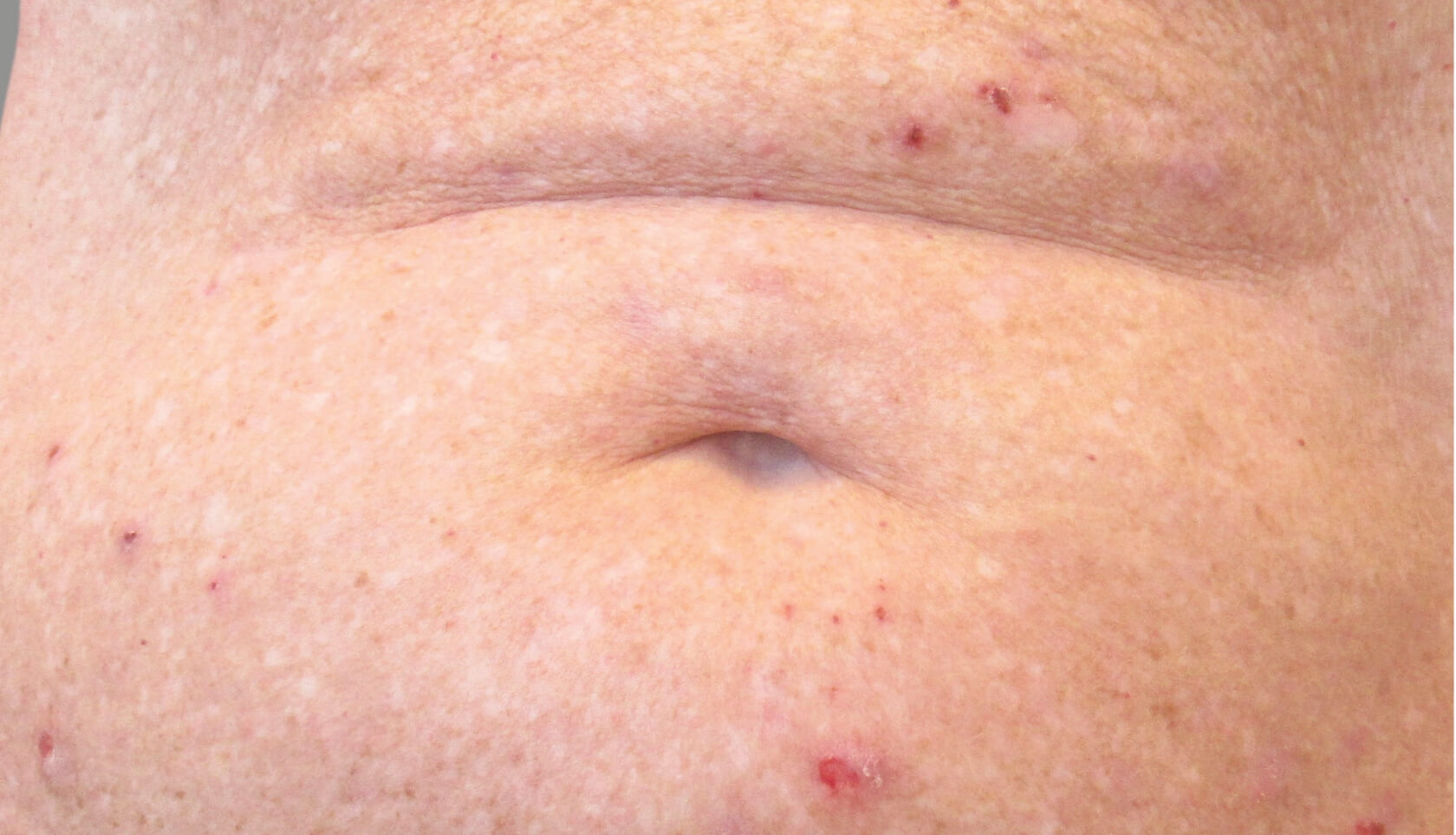
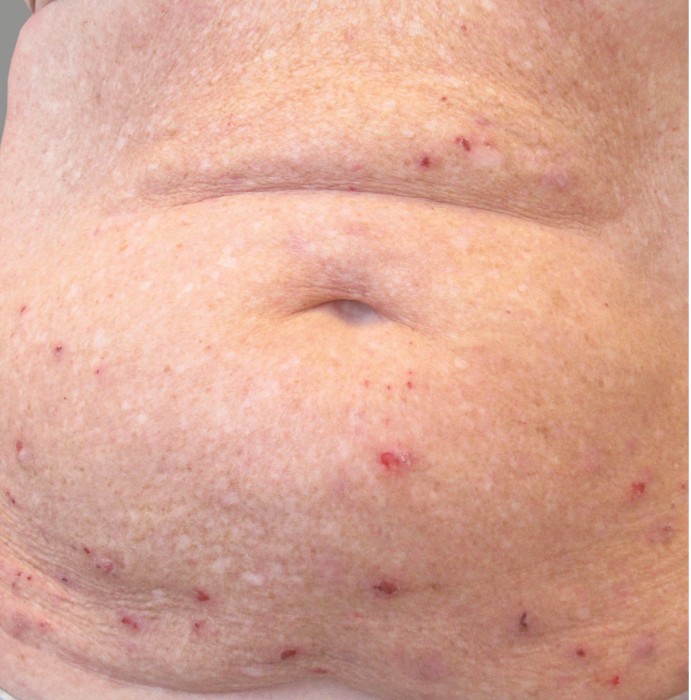
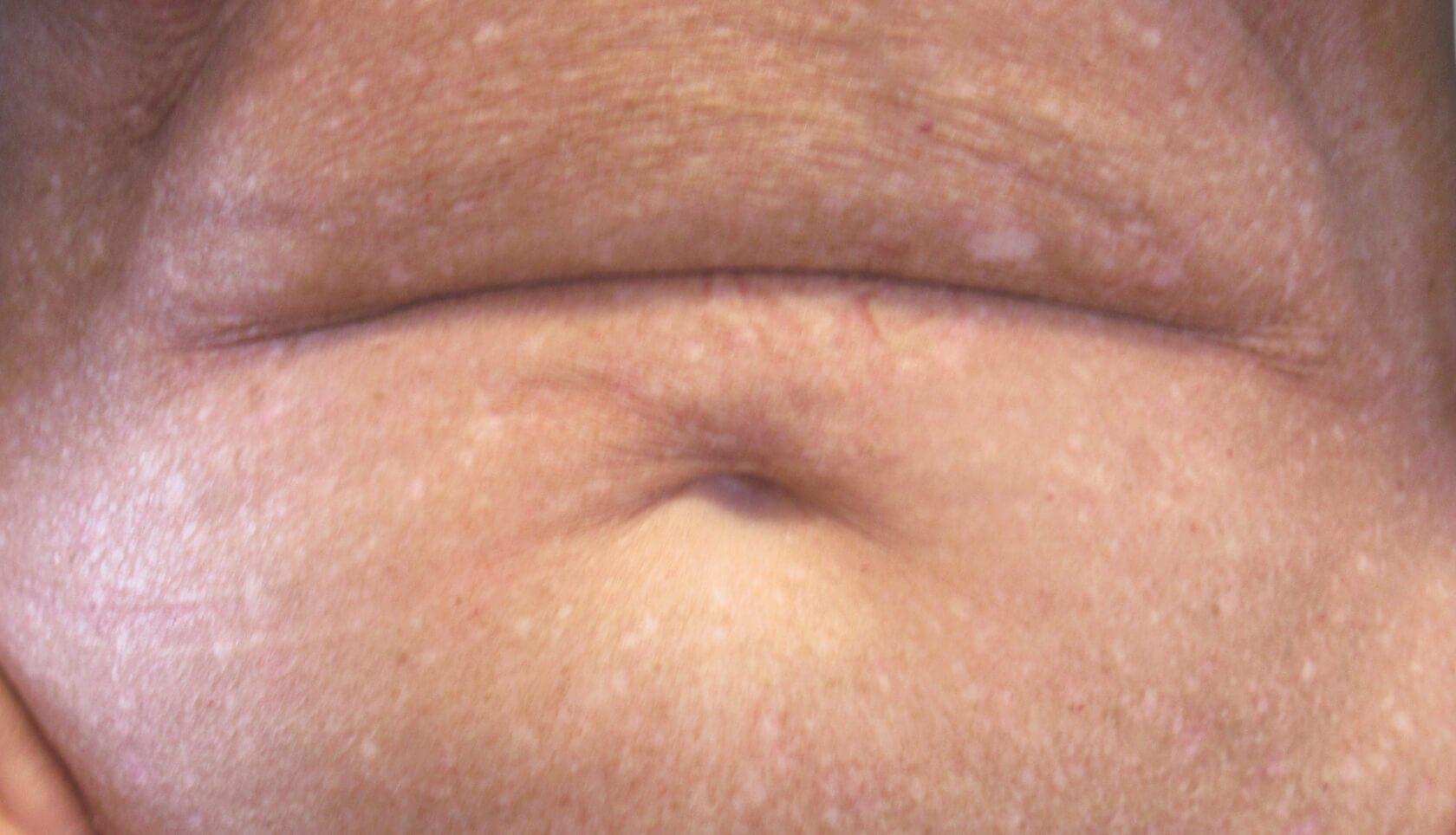
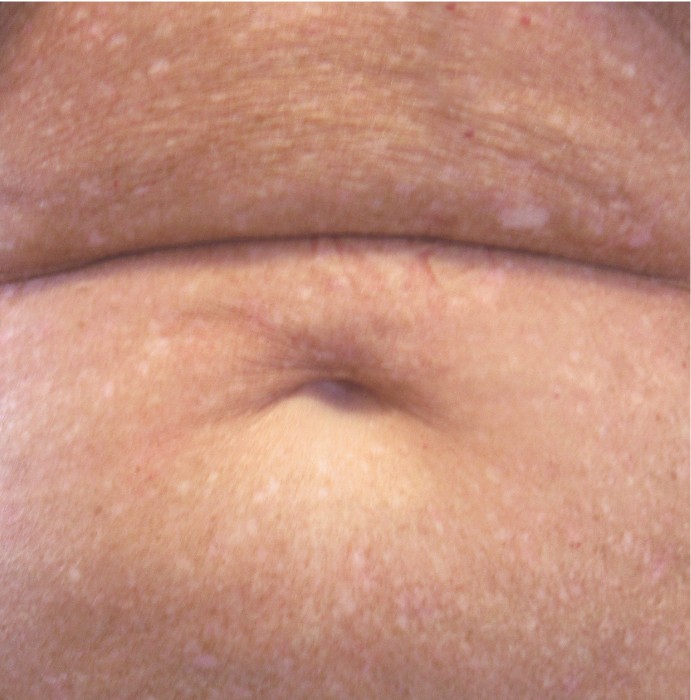
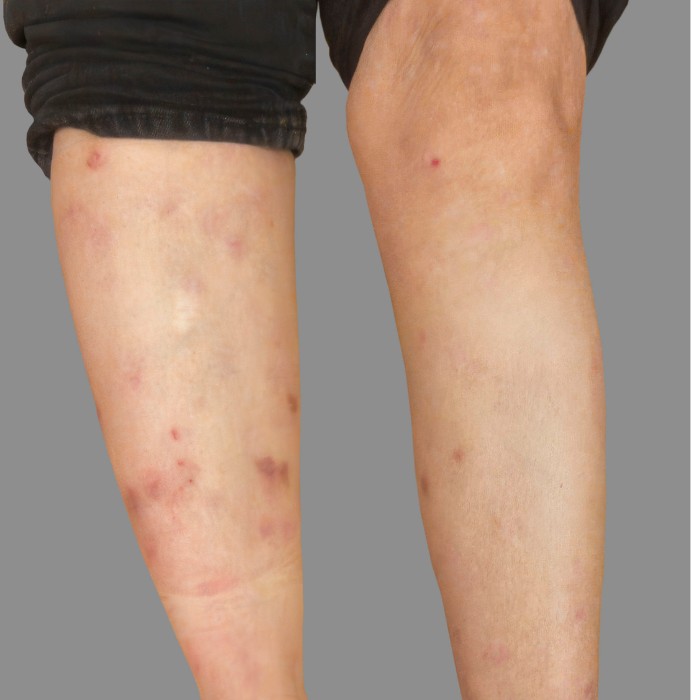
PATIENT 2: 79-YEAR-OLD MALE PATIENT WITH A
2-POINT IMPROVEMENT IN IGA PN-S
Current PN therapy: DUPIXENT 300 mg Q2W
RESULTS
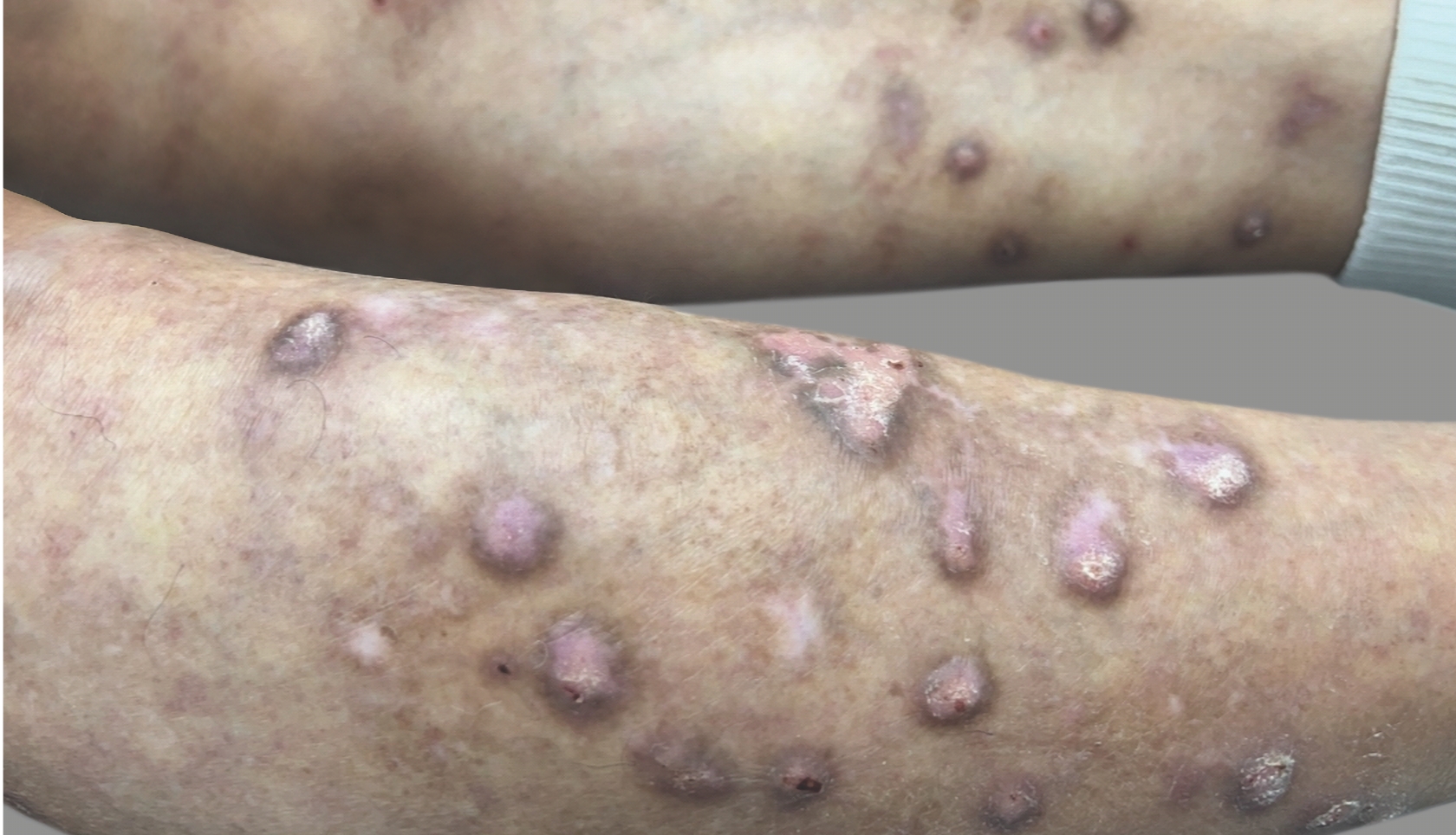
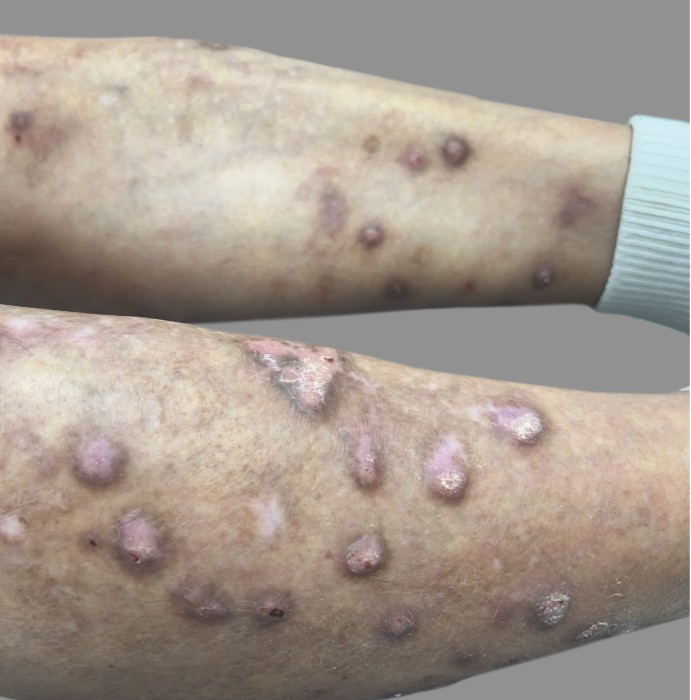
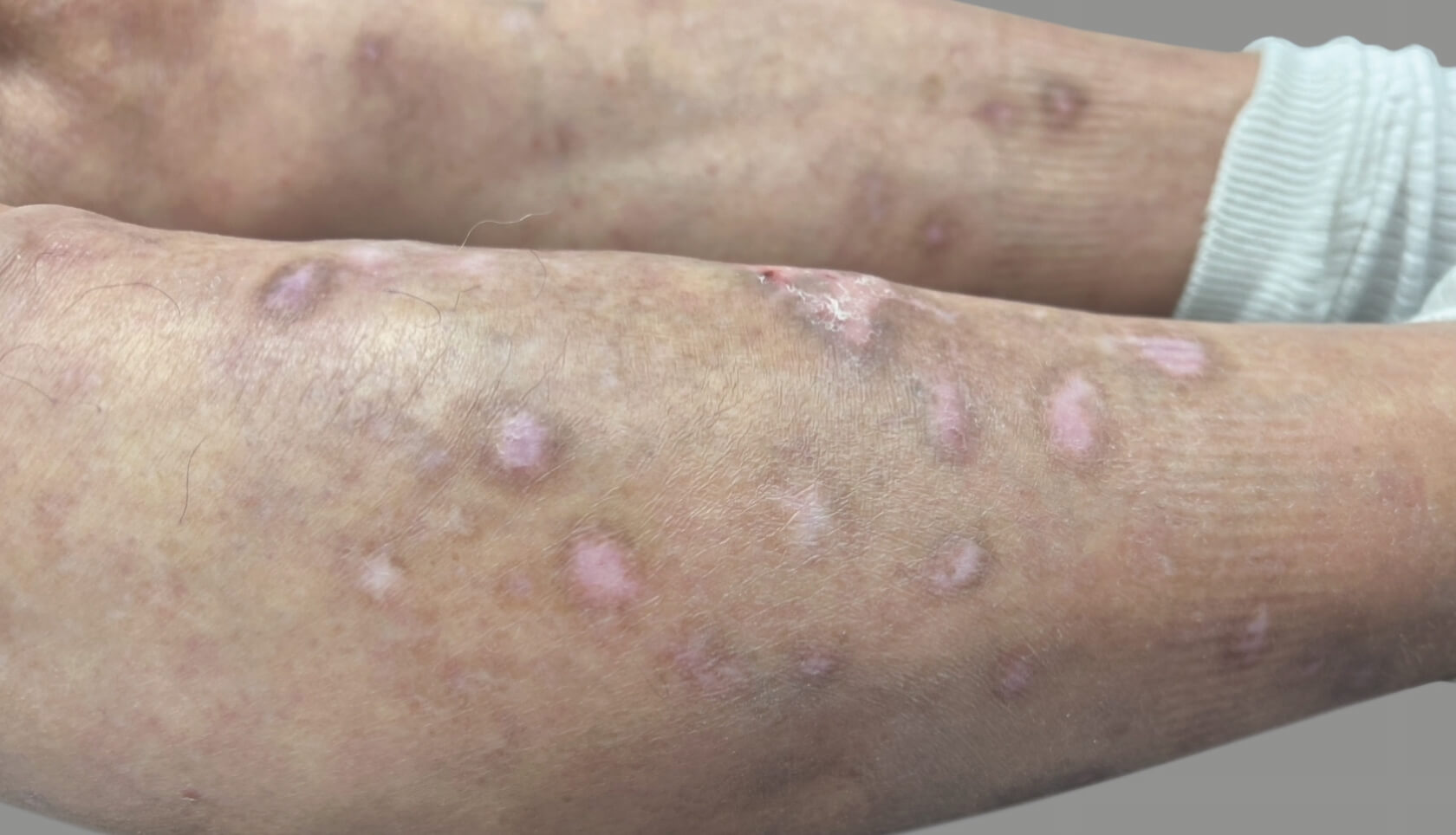
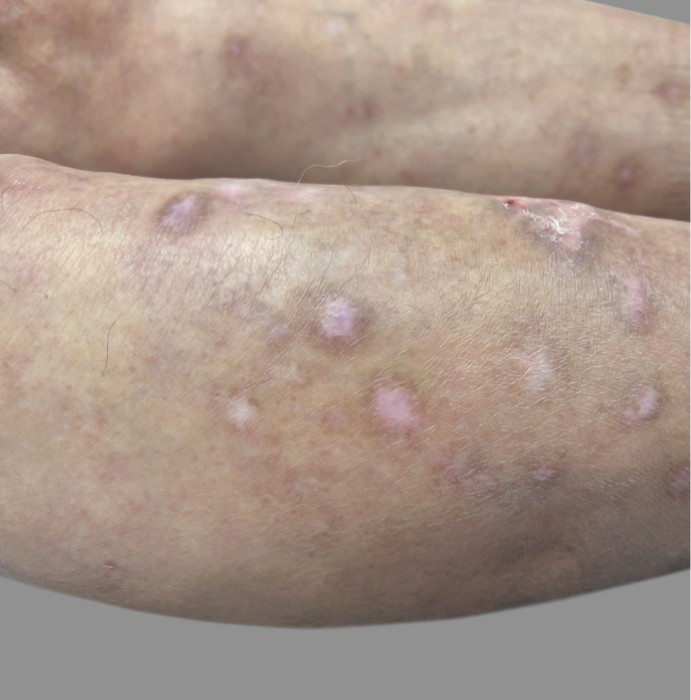
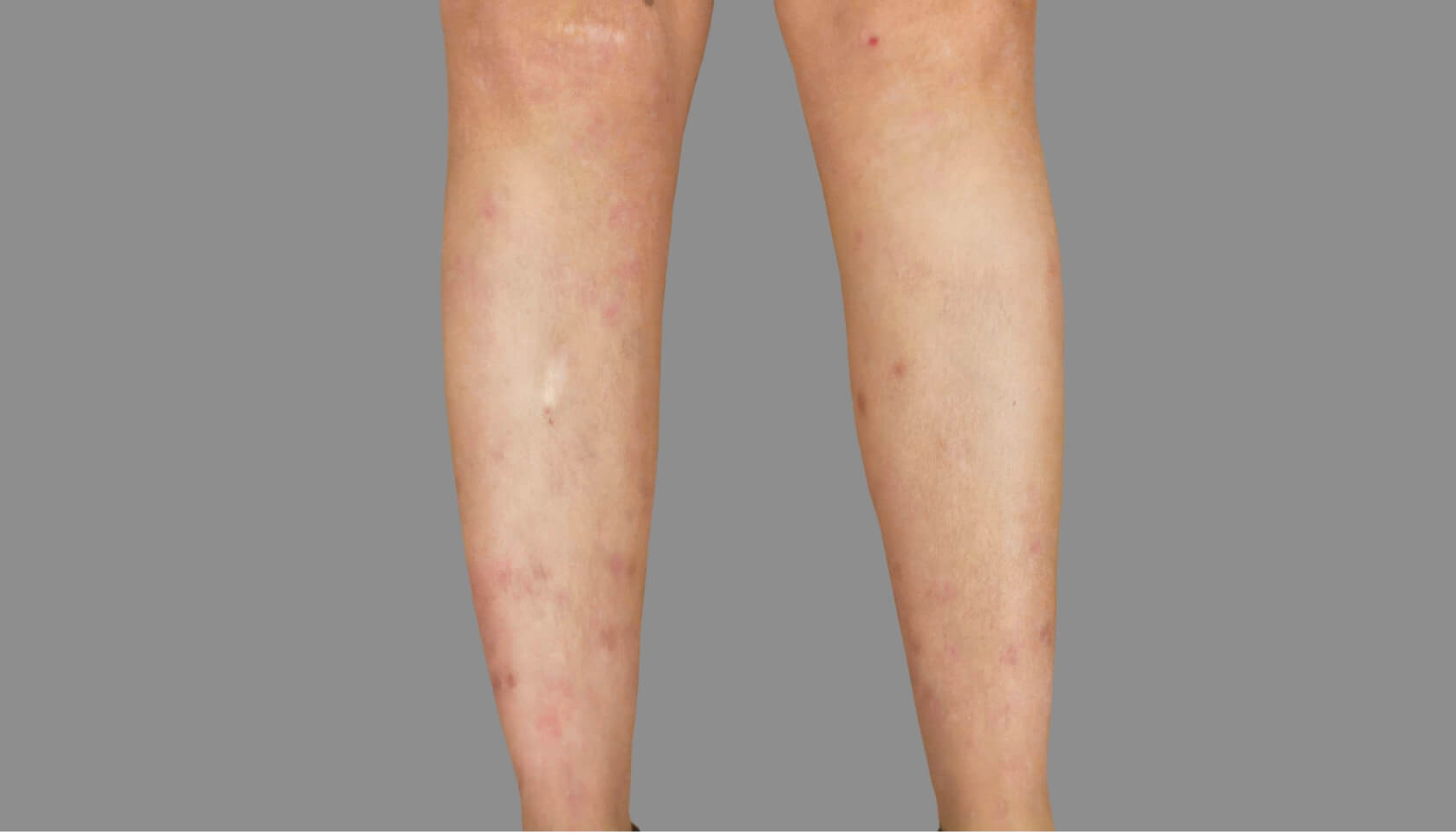
PATIENT 3: 53-YEAR-OLD FEMALE PATIENT WITH A
2-POINT IMPROVEMENT IN IGA PN-S
Current PN therapy: DUPIXENT 300 mg Q2W
RESULTS
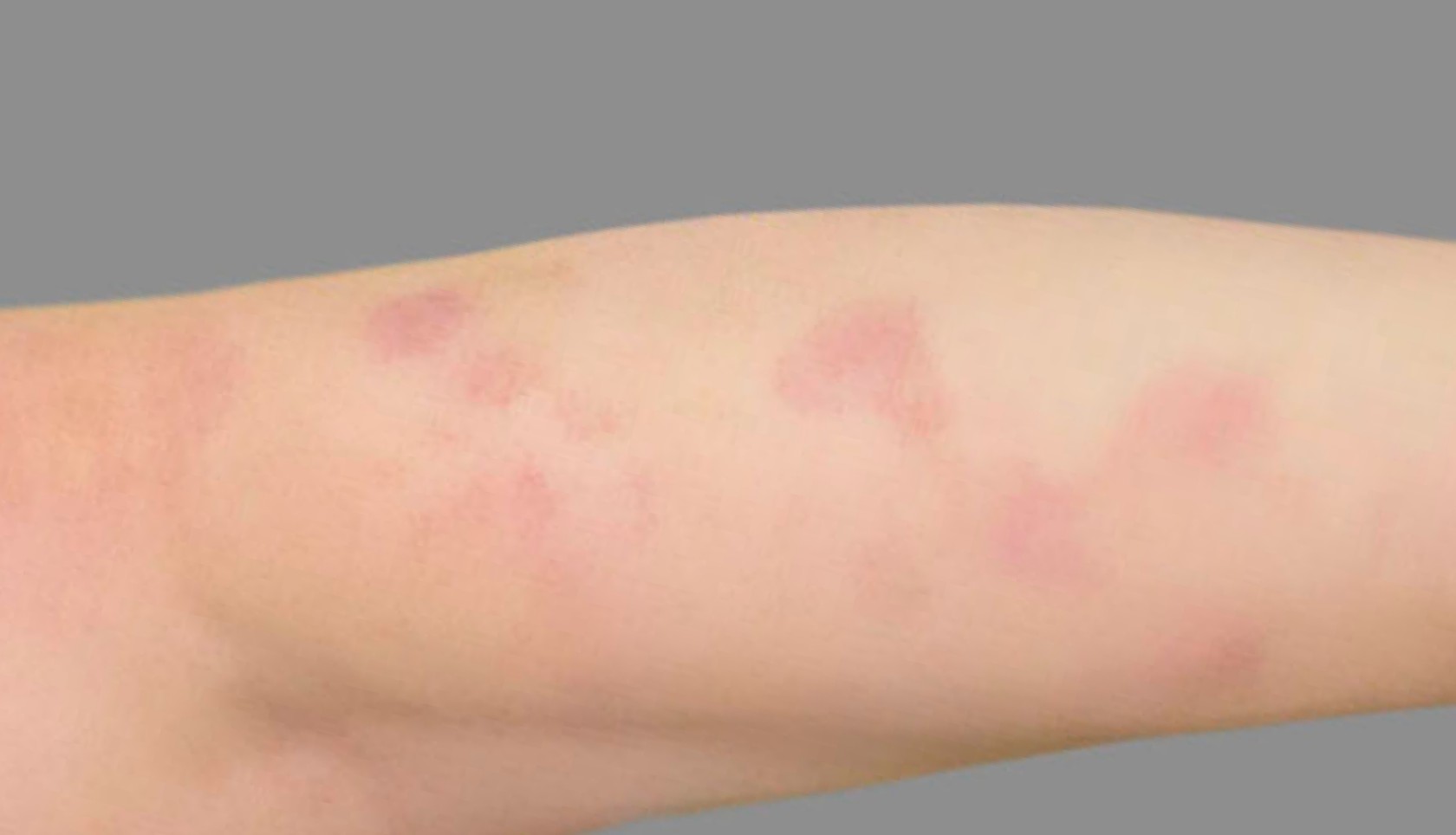
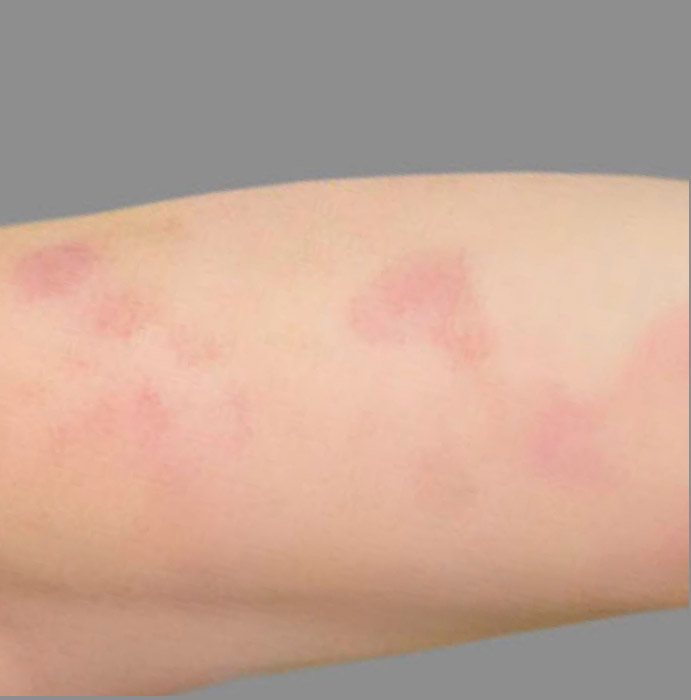
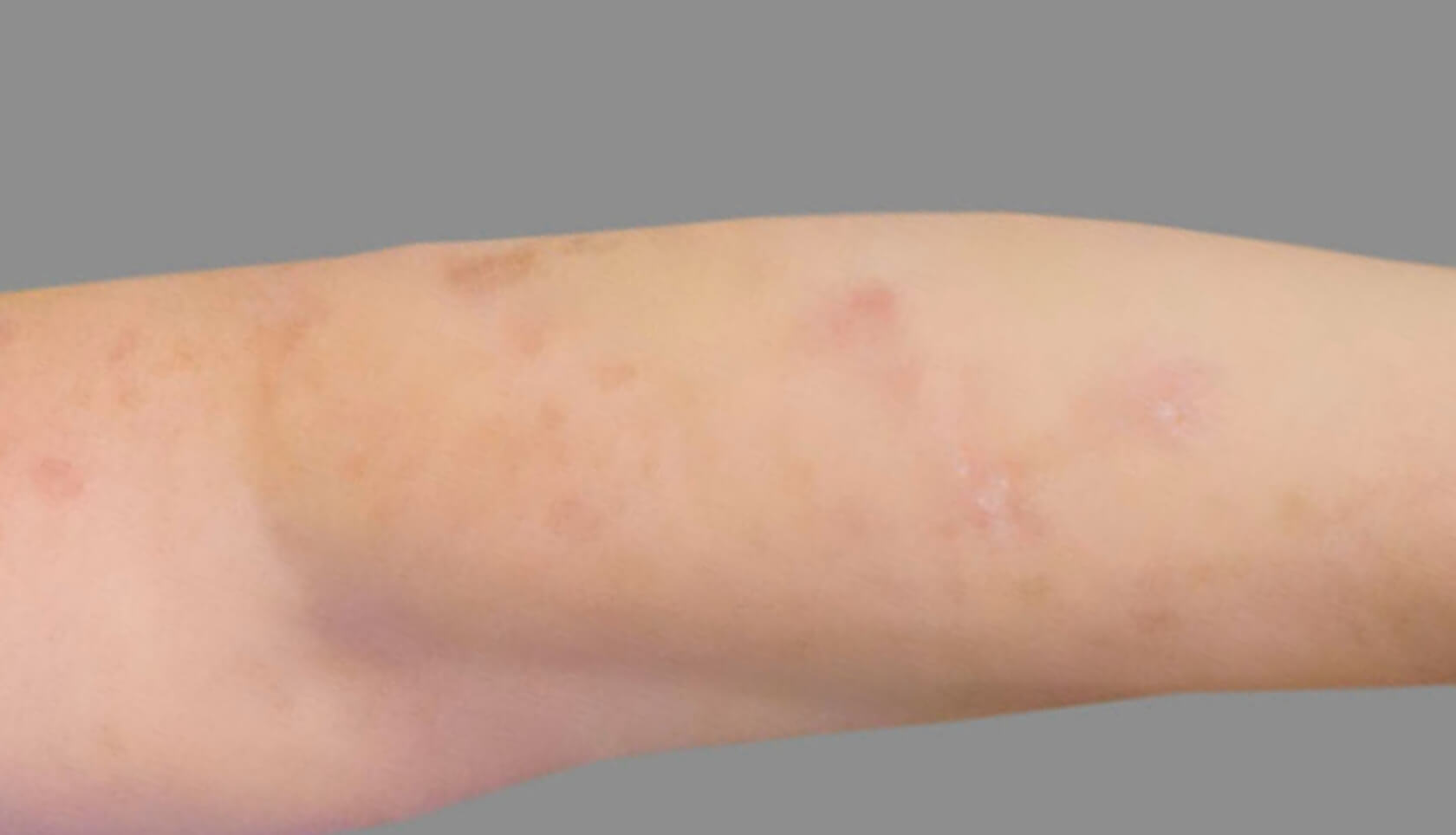
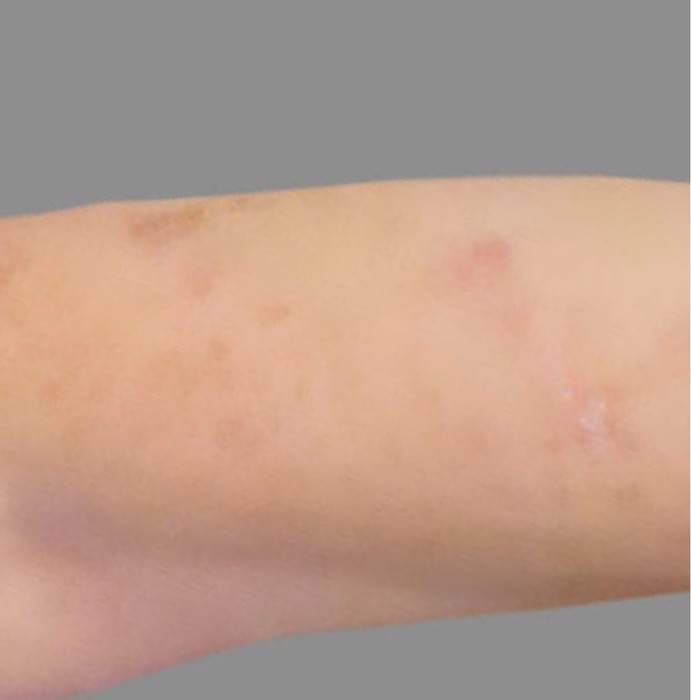
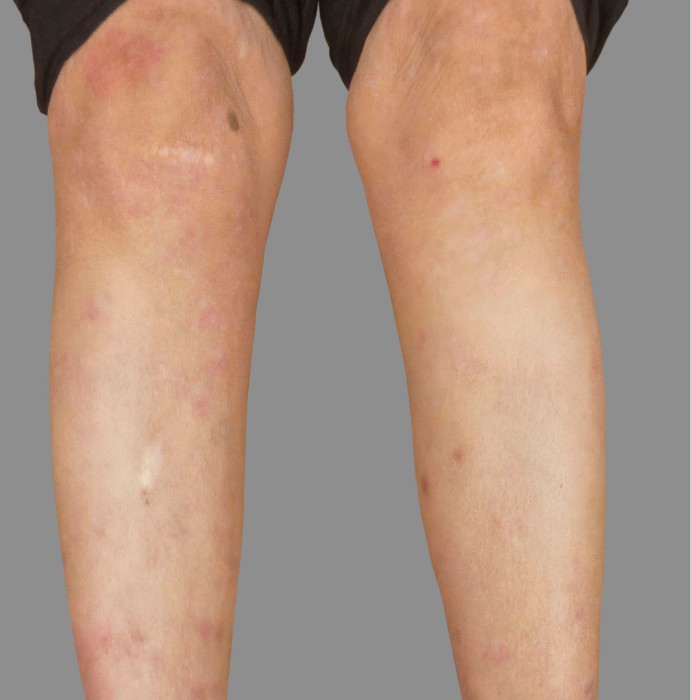
PATIENT 4: 53-YEAR-OLD FEMALE PATIENT WITH A
2-POINT IMPROVEMENT IN IGA PN-S
Current PN therapy: DUPIXENT 300 mg Q2W
RESULTS
PROPORTION OF PATIENT
WITH
IGA PN-S
0 OR 1 AT
WEEK 24
(secondary endpoint)1-3,a
aBaseline IGA PN-S scores: 3.3 (SD, 0.5) for DUPIXENT, 3.3 (SD, 0.5) for placebo.
- A nominal difference was observed at Week 4 (9% with DUPIXENT vs 1% with placebo) and at Week 12 (32% with DUPIXENT vs
12% with placebo)3
Definitive conclusions cannot be made for results earlier than 24 weeks in PRIME. Data were not multiplicity controlled.
- At Week 4, 8% of patients treated with DUPIXENT vs 6% with placebo had nodule clearance. A significantly greater proportion of DUPIXENT patients achieved nodule clearance at Week 12 (26% with DUPIXENT vs 12% with placebo; P=0.019, secondary endpoint) and Week 24 (45% with DUPIXENT vs 16% with placebo; P<0.001, secondary endpoint)1-3
Definitive conclusions cannot be made for results at timepoints other than Week 12 and 24 in PRIME2. Data were not multiplicity controlled.
DUPIXENT DEMONSTRATED SIGNIFICANT
NODULE CLEARANCE AT WEEK 24
to see results


DUPIXENT DEMONSTRATED SIGNIFICANT
NODULE CLEARANCE AT WEEK 24
RESULTS
Illustrative representation of nodule clearance based on clinical trial results. Actual individual results may vary.
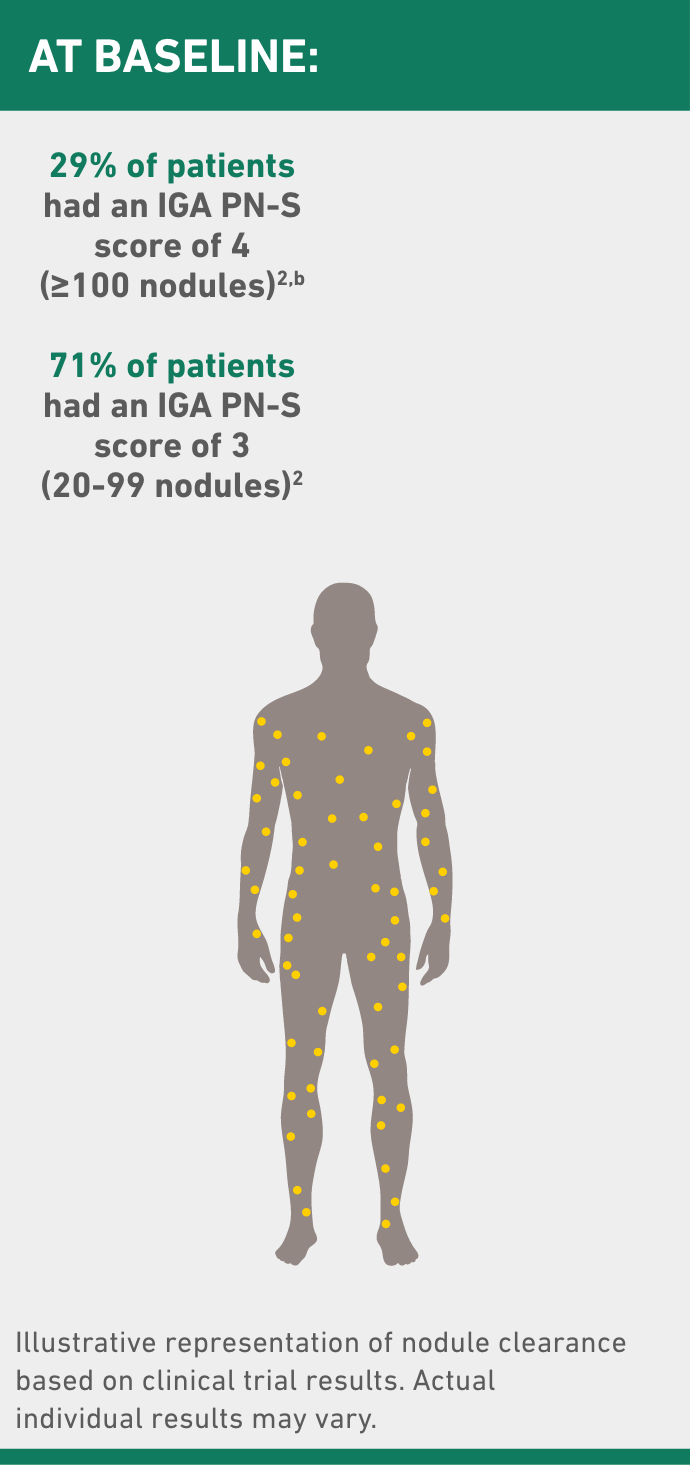
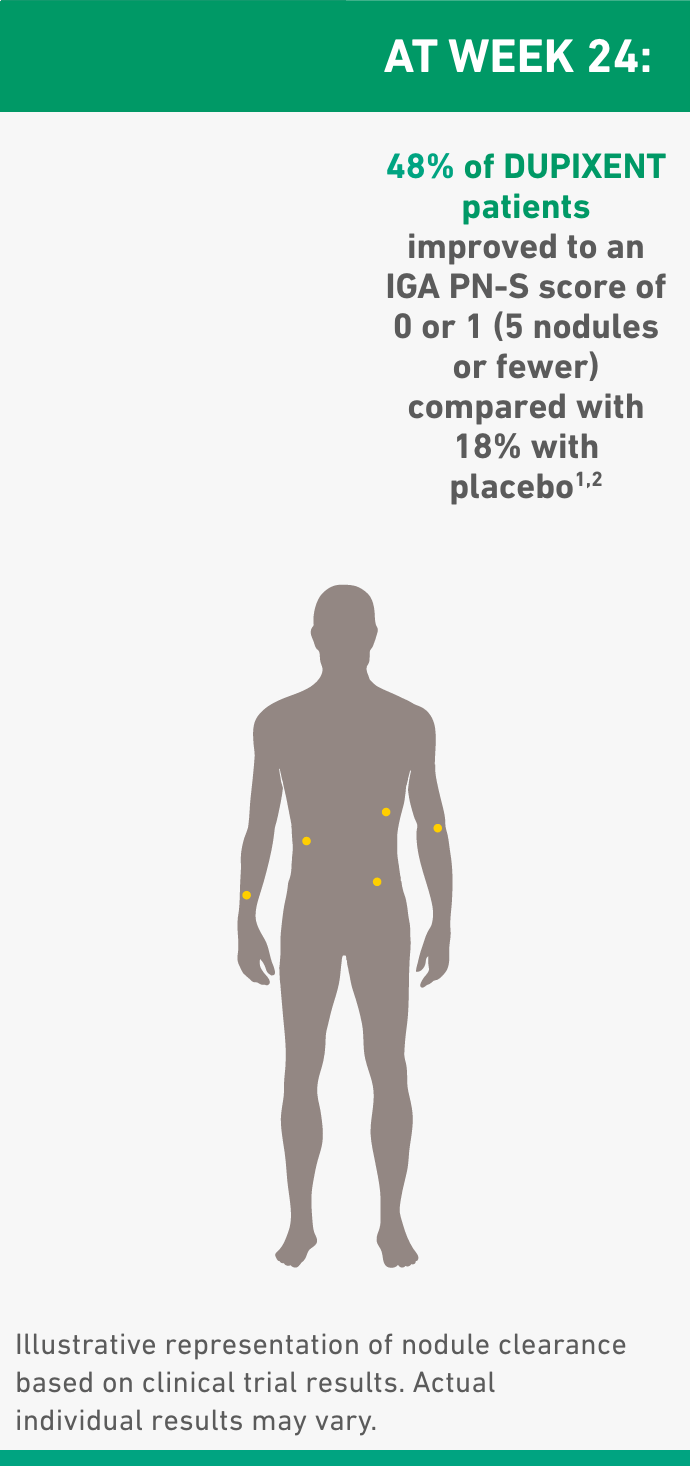
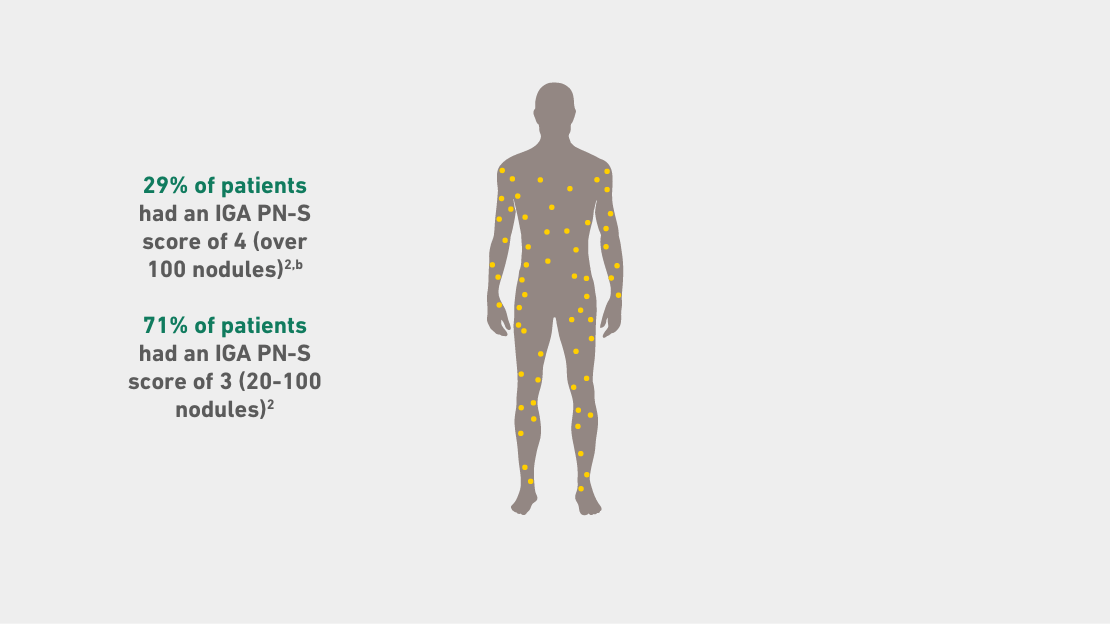
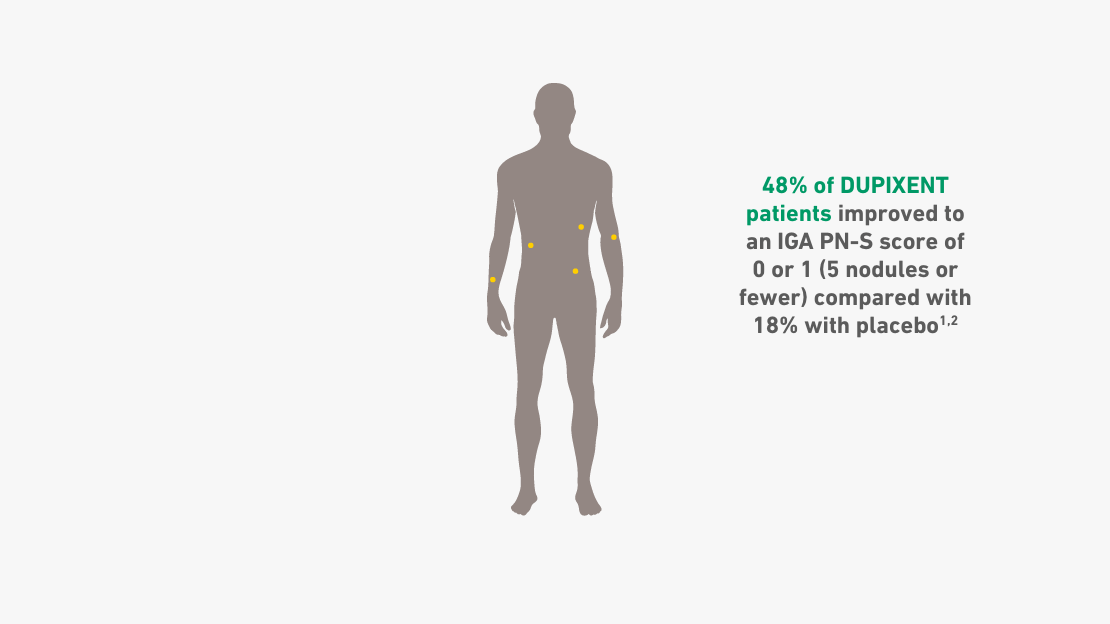
IGA PN-S ranges from 0 (clear, no nodules) to 1 (almost clear, ≤5 nodules), 2 (mild, 6-19 nodules), 3 (moderate, 20-99 nodules), and 4 (severe, ≥100 nodules).
18+ YEARS
- 38% of patients had 100 or more nodules at baseline, with 45% of all DUPIXENT patients achieving 5 nodules or fewer at Week 24 vs 16% with placebo1,2
bAll study participants had ≥20 nodules upon screening and at Day 1 (inclusion criteria).
DUPIXENT showed consistent efficacy regardless of patients’ atopic
background1,4
PRIME and PRIME2
Nodule improvement regardless
of patients’ history of atopy
Proportion of patients with IGA PN-S
score of 0 or 1 at Week 241,4
Definitive conclusions cannot be made for these results as the data were not
multiplicity controlled and P values were nominal.
In the PRIME trials, patients were stratified by their history of atopy.
IGA PN-S, Investigator’s Global Assessment PN-Stage; PN, prurigo nodularis; Q2W, once every 2 weeks; SD, standard deviation.
EXPLORE COMPOSITE ENDPOINT