REVOLUTIONIZING THE
TREATMENT FOR PN
- First and only therapy to target
IL-4Rα, thereby specifically inhibiting
IL-4 and IL-13 signaling1
The mechanism of dupilumab action has not been definitively established.
SEE HOW IT WORKSDUPIXENT is indicated for the treatment of adult patients with prurigo nodularis (PN).
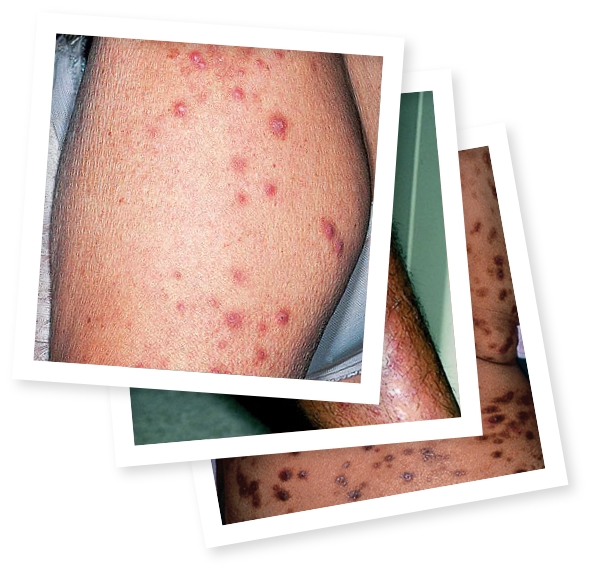
JENNIFER HAS IMPROVED ITCH AND NODULE
CLEARANCE AS HER CREATIONS BLOOM
In the us, studied in 17 pivotal trials across 5 indications2
Globally,
≈800,000
patients on DUPIXENT
therapy across 5
indications3,a
aThe worldwide patient number is largely comprised of patients treated with DUPIXENT from 10 countries (Canada, China, France, Germany, Italy, Japan, the Netherlands, Spain, UK, and US) and the rest of the world comprising ≈10% of this worldwide patient number. Data through December 2023.
The mechanism of dupilumab action has not been definitively established.
SEE HOW IT WORKSaccording to the Prescribing Information1
Please see additional Warnings and
Precautions in the Prescribing Information
and Important Safety Information throughout. below.
Not metabolized through the liver or excreted through the kidneys
Select important safety information
WARNINGS AND PRECAUTIONS
Hypersensitivity: Hypersensitivity reactions, including anaphylaxis, acute generalized exanthematous pustulosis (AGEP), serum
sickness
or serum sickness-like reactions, angioedema, generalized urticaria, rash, erythema nodosum, and erythema multiforme have been
reported. A case of AGEP was reported in an adult subject who participated in the bullous pemphigoid development program. If a clinically
significant hypersensitivity reaction occurs, institute appropriate therapy and discontinue DUPIXENT.
BY DERMATOLOGISTS2,a
On therapy across approved
indications worldwide2,b
among specialty systemic
therapies indicated
in PN2,c-e
FDA-approved for 4 indications in dermatology
Most efficient path to starting treatment for most patientsf ≈98% of commercial PN patients (18+ years of age) nationally are covered for DUPIXENT
What does PN look like on different skin tones?