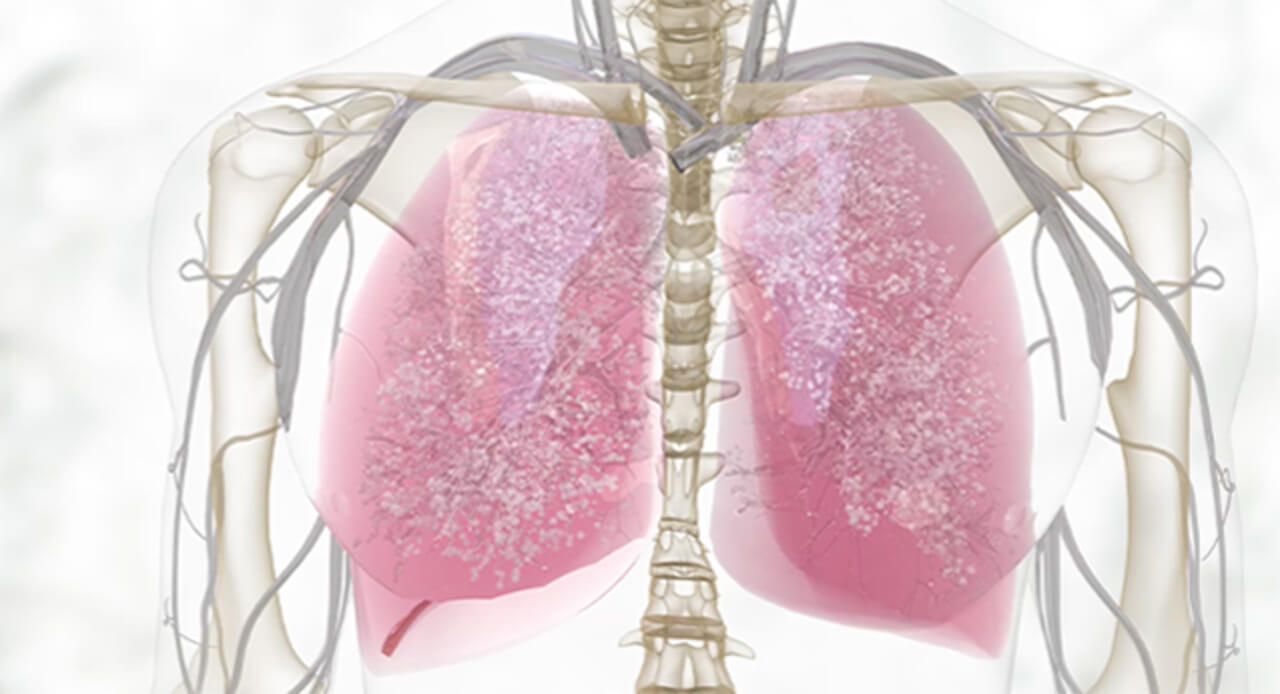
Discover helpful
resources to further
understand DUPIXENT
Watch and learn more about DUPIXENT
MOA OF DUPIXENT
See how DUPIXENT inhibits IL-4 and IL-13 signaling
What are two of the key sources of local type 2 inflammation in asthma?
Patients who suffer with asthma may be sensitive to potential triggers that can initiate an asthma exacerbation. Let’s take a deep look inside the lower respiratory epithelium to see what happens during an asthma exacerbation. When patients with asthma encounter potential triggers, this can lead to epithelial inflammation and alarmin activation. This trigger can also lead to signaling of Th2 cells and ILC2s which release cytokines such as IL-4, IL-5 and IL-13. Let’s look inside the epithelium to focus on IL-13 and its role in type 2 inflammation. IL-13 is a key source of local type 2 inflammation. When activated, it contributes to epithelial barrier dysfunction, eosinophilic inflammation, mucus overproduction and smooth muscle contraction.
How do eosinophils contribute to systemic type 2 inflammation in asthma?
IL-4 is a key source of systemic type 2 inflammation that causes eosinophil trafficking to the site of inflammation. Eosinophils release eosinophilic cationic protein or ECP, eosinophil peroxidase or EPO, and other chemical mediators that contribute to asthma pathology. IL-4 also causes B cell activation, IGE production, as well as mass cell and basophil activation, and trafficking. IGE binds to receptors on the surface of mast cells and basophils and cross links with antigen. This results in mast cell and basophil degranulation releasing additional cytokines, histamines and leukotrienes.
Did you know that DUPIXENT targets two of the sources of type 2 inflammation?
DUPIXENT is the only dual-inhibitor of IL-4 and IL-13 signaling, two of the key drivers of local and systemic type 2 inflammation. The mechanism of dupilumab action has not been definitively established. There are two types of receptors blocked by DUPIXENT: the type 1 receptor and the type 2 receptor. Type 1 receptors are located within the cell membranes of mast cells, basophils, eosinophils, neutrophils and T cells. Type 2 receptors are located within the cell membranes of epithelial cells, smooth muscle cells and fibroblasts. DUPIXENT binds to the IL-4 receptor blocking IL-4 and IL-13 intracellular signaling. Pharmacodynamics data with DUPIXENT showed a decrease in total IGE and a transient increase in blood eosinophils. DUPIXENT also prevents complexing of IL-13 receptor with the IL-4 receptor complex. Pharmacodynamics data with DUPIXENT showed decreased FeNO levels. In clinical trials, patients with uncontrolled asthma, treated with DUPIXENT experienced up to 35% reduction from baseline at week 96 in blood eosinophils, up to 83% reduction from baseline at week 96 in total IGE, and up to 35% reduction from baseline in FeNo after two weeks. Correlations between pharmacodynamics data and efficacy endpoints have not been established. Results are descriptive.
Make DUPIXENT your first-choice biologic for your appropriate patients with an eosinophilic phenotype or OCS-dependent asthma, driven by type 2 inflammation.
DUPIXENT MyWay is a patient support program that can help enable access to DUPIXENT and offers financial assistance for eligible patients, one‑on‑one nursing support, and more.
WATCH INFORMATIONAL
VIDEOS ABOUT:
Contact a Field Representative
Connect with a DUPIXENT Field Representative to get answers to your product-related questions and to request samples.