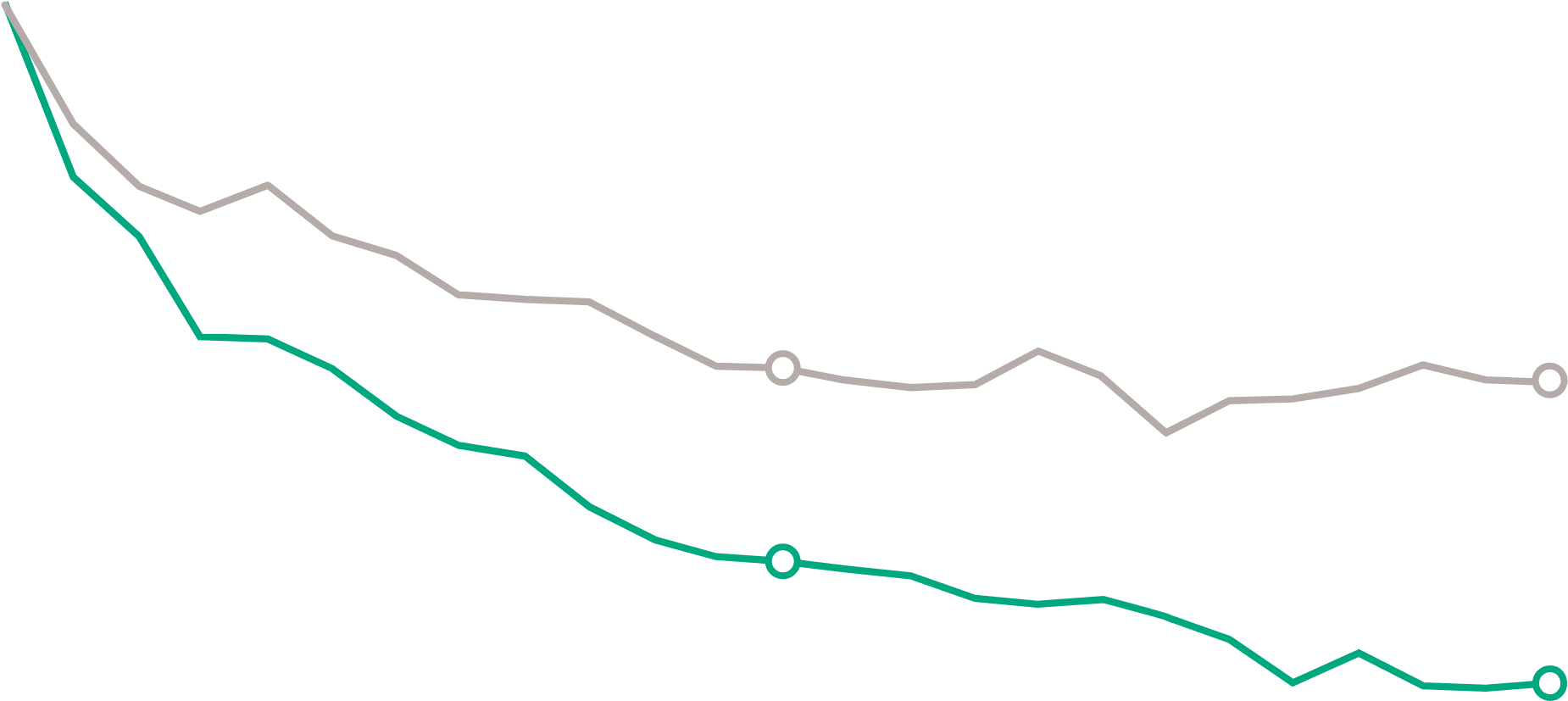
HSS7 - WEEKLY HIVES
(Week 24, secondary endpoint)1,2
Mean HSS7 at baseline—DUPIXENT: 15.85 (SD, 3.76); placebo: 15.03 (SD, 4.81).2
- Nominal difference observed at Week 12 (DUPIXENT: -8.60 vs placebo: -5.65)
hierarchical testing procedure.
vs 31% reductiona in HSS7 at Week 24
(DUPIXENT: -7.16 vs placebo: -5.15; P=0.0450, secondary endpoint)1,2
- Reductiona in HSS7 at Week 12 (DUPIXENT: -5.62 vs placebo: -4.31, secondary endpoint; not significant)2
- Mean HSS7 at baseline: DUPIXENT (n=73): 13.31 (SD, 4.43); placebo (n=75): 12.90 (SD, 4.98)1,2
Definitive conclusions cannot be made for Week 12 as the analysis was not multiplicity controlled.
aLSM change from baseline.
HSS7, hive severity score over 7 days; LSM, least squares mean; SD, standard deviation; UAS7, urticaria activity score over 7 days.
Review more details
Hives severity score (HSS) is a patient-reported assessment rated on a scale of 0 (none), 1 (<20 hives), 2 (20 to 50 hives), and 3 (>50 hives). HSS7 is the sum of the preceding 7 days’ hives scores, for a total HSS7 range of 0 to 21.2
SEE WHICH PATIENTS WITH CSU MAY BE APPROPRIATE
FOR DUPIXENT