uaS7 - WEEKLY disease activity
(UAS7=ISS7 + HSS7; Week 12 and Week 24, secondary endpoint)1,2
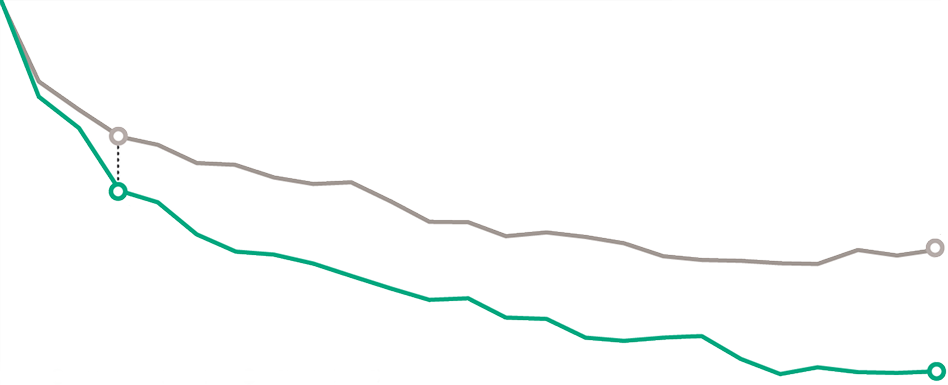
Mean UAS7 at baseline—DUPIXENT: 32.10 (SD, 7.01); placebo: 30.76 (SD, 8.19).2
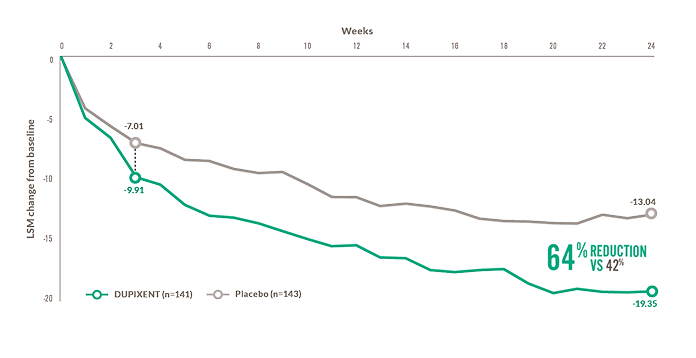
vs 32% reductiona in UAS7 at Week 24
(DUPIXENT: -15.61 vs placebo: -11.27; P=0.0324, secondary endpoint)1,2
- 36% vs 27% reductiona in UAS7 at Week 12 (DUPIXENT: -12.59 vs placebo: -9.65, secondary endpoint; not significant)2
- Mean UAS7 at baseline: DUPIXENT (n=73): 28.48 (SD, 7.10); placebo (n=75): 27.86 (SD, 7.82)1,2
Definitive conclusions cannot be made for Week 12 as the analysis was not multiplicity controlled.
aLSM change from baseline.
IMPROVEMENT IN UAS7 WAS OBSERVED AS EARLY
AS WEEK 3 IN A POST HOC POOLED ANALYSIS
cupid-a and cupid-c pooled analysis2
CUPID-C
Mean UAS7 at baseline—DUPIXENT: 30.23 (SD, 7.26); placebo: 29.24 (SD, 8.10).2
Definitive conclusions cannot be made as this was a post hoc analysis which was not multiplicity controlled.
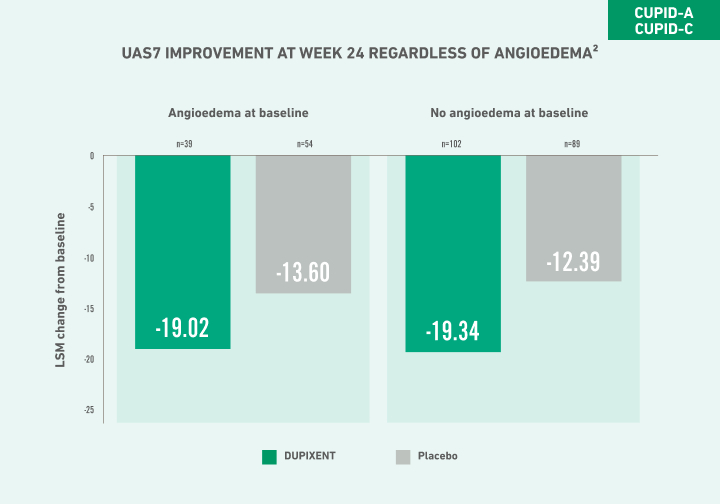
IN POST HOC POOLED ANALYSES OF CUPID-A AND CUPID-C, IMPROVEMENT IN UAS7:
At Week 24 was consistent regardless of patients’ baseline IgE1,2
At Week 24 was generally consistent regardless of baseline BMI2,b
Definitive conclusions cannot be made as these were post hoc analyses which were not multiplicity controlled.
bAcross BMI stratifications: BMI (kg/m2) <25, ≥25 to <30, and ≥30.
BMI, body mass index; HSS7, hives severity score over 7 days; ISS7, itch severity score over 7 days; LSM, least squares mean; SD, standard deviation; UAS7, urticaria activity score over 7 days.
See data for itch and hives separately
Urticaria activity score (UAS) is a patient-reported assessment of urticaria severity. UAS7 is the sum of the preceding 7 days’ itch severity score (ISS) and hives severity score (HSS), for a total UAS7 range of 0 to 42.2
ISS7
+
HSS7
=
UAS7
SEE WHICH PATIENTS WITH CSU MAY BE APPROPRIATE FOR DUPIXENT