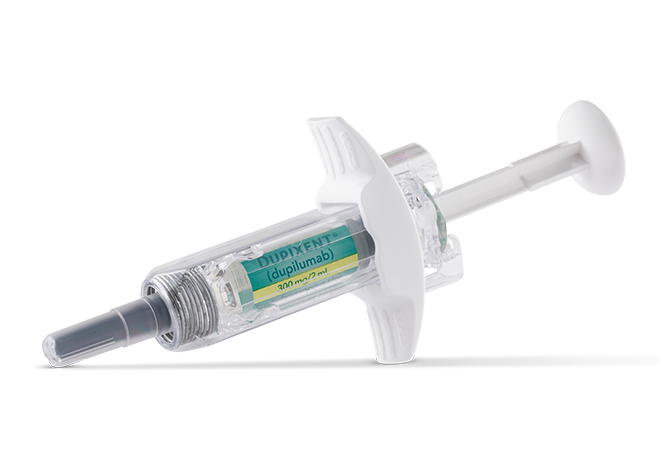
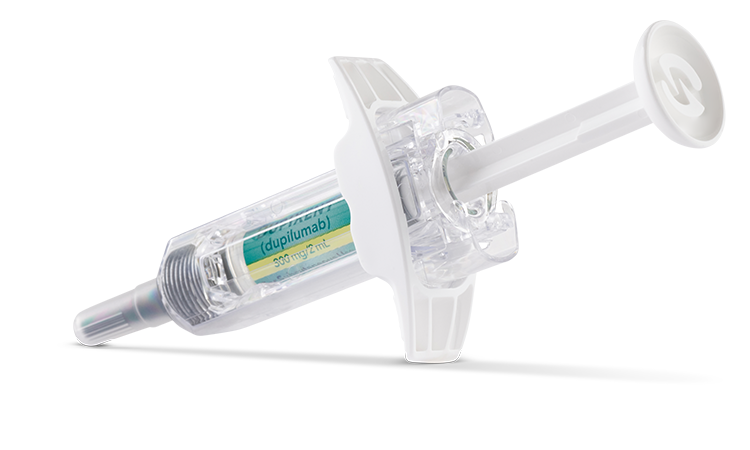
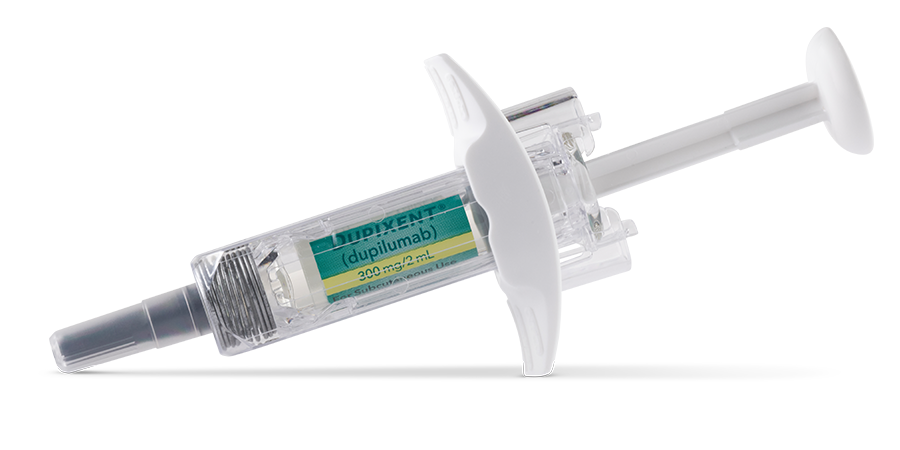
DOSAGE FOR ADULTS AND ADOLESCENTS (12+ YEARS)1
Adults and adolescents (12+ years) with inadequately controlled CRSwNP1
12+ YEARS
No loading dose
300 mg once every
two weeksa
1 pre-filled pen or syringe
a300 mg=2 mL solution.1
For supplemental injection training, visit our Patient Video Injection Support page.
Two Administration Options for You and Your Patients1-3
Consider Before Injecting1
DUPIXENT is a medicine that is administered by subcutaneous injection and is intended for use under the guidance of a
healthcare provider.1
- In children 12 years of age and older, it is recommended that DUPIXENT be administered by or under the supervision of an adult
- A patient may self-inject DUPIXENT—or a caregiver may administer DUPIXENT—after training has been provided by a healthcare
provider on proper subcutaneous injection technique using the pre-filled syringe or pre-filled pen - It is important to provide proper training to patients and/or caregivers on the preparation and administration of DUPIXENT prior to use
- Advise patients to follow sharps disposal recommendations after administration
Important Administration Instructions1
- Patients and/or caregivers should read the Instructions for Use prior to injecting
- Instruct patients and/or caregivers to administer the subcutaneous injection
into the thigh or abdomen, except for the 2 inches (5 cm) around the navel - The upper arm can also be used if a caregiver administers the injection
- It is important to rotate the injection site with each injection. DO NOT inject
DUPIXENT into skin that is tender, damaged, bruised, or scarred - If a dose is missed, instruct the patient to administer the injection within
7 days from the missed dose and then resume their original schedule. If the
missed dose is not administered within 7 days, instruct the patient to administer the dose, starting a new schedule based on this date
Pre-filled Pen
Additional Dosing Information
Before injection, instruct patients and/or caregivers to remove DUPIXENT from the refrigerator and allow DUPIXENT to reach room temperature without removing the needle cap.
- 45 minutes for the 300 mg/2 mL
pre‑filled syringe or pen
Patients and/or caregivers should inspect DUPIXENT for particulate matter and discoloration prior to administration.
- Do not use if the liquid contains visible particulate matter or is discolored or cloudy (other than clear to slightly opalescent, colorless to pale yellow)
Patients and/or caregivers should discard any unused product remaining in the pre‑filled syringe or pen in accordance with local requirements.
- DUPIXENT is sterile and preservative‑free. Patients and/or caregivers should discard any unused portion
- Patients and/or caregivers should store DUPIXENT by refrigerating between 36 °F and 46 °F (2 °C and 8 °C) in the original carton to protect from light
- If necessary, DUPIXENT may be kept at room temperature up to 77 °F (25 °C) for a maximum of 14 days. DUPIXENT should not be stored above 77 °F (25 °C). After removal from the refrigerator, DUPIXENT must be used within 14 days or discarded
- DUPIXENT should not be exposed to heat or direct sunlight
- DO NOT freeze. DO NOT shake
- Any unused medicinal product or waste material should be disposed of in accordance with local requirements
DUPIXENT MyWay®
Get patient access support and information about benefits investigations, prior authorizations, and medical necessity and appeal letters.
)