Do you have CRSwNP patients like these?
Consider DUPIXENT for patients with uncontrolled CRSwNP who have any of the following signs of type 2 inflammation1-10:
Repeated systemic steroid bursts and/or history of sinus surgery2,3
Loss of smell2,4-6
Nasal congestion2,4,5
Polyp recurrence7,8
History of diseases driven in part by type 2 inflammation5,9,10
UP TO 87%
of patients with CRSwNP have
evidence of type 2 inflammation11
DUPIXENT offers an approach
to address a cause of type 2
inflammation, making it an important
treatment option for my patients.1,12,13,*
Nicole Chase, MD, FAAP, FAAAAI, FACAAI
Allergist/Immunologist, Partner,
St. Paul Allergy & Asthma, P.A.
Associate Professor, University of
Minnesota School of Medicine
*The mechanism of dupilumab action has
not been definitively established.1
Explore Some of the Possible Patient Types Right for DUPIXENT

-
RAPID RECURRENCE
MEET RAQUEL
Symptoms returned only a few months post surgery
IDENTIFIED EARLY SIGNS OF RECURRENCE? THINK DUPIXENT1,6,14,15
Raquel, 36rapid RECURRENCEPatient profile is representative and is not an actual DUPIXENT patient. CLINICAL PROFILE
CLINICAL PROFILE- Diagnosed with CRSwNP 9 months ago and prescribed INCS
- Underwent sinus surgery 6 months ago; reported loss of smell at 3-month follow-up
- Nasal congestion, facial pressure, and polyp growth have reappeared 6 months post surgery
LOSS OF SMELL
may be the first sign of recurrence after sinus surgery—
and is associated with type 2 inflammation.2,4-6,14Raquel, 36rapid RECURRENCE![]() Patient profile is representative and is not an actual DUPIXENT patient.
Patient profile is representative and is not an actual DUPIXENT patient.
-
STEROID CYCLER
MEET BEN
Multiple rounds of oral steroids 2 years post surgery
TARGET TWO OF THE KEY SOURCES OF TYPE 2 INFLAMMATION WITH DUPIXENT1,12,13,*
*The mechanism of dupilumab action has not been definitively established.1
Ben, 31steroid cyclerPatient profile is representative and is not an actual DUPIXENT patient. CLINICAL PROFILE
CLINICAL PROFILE- Diagnosed with CRSwNP and prescribed INCS
- Underwent sinus surgery 2 years ago
- History of childhood asthma
- Recent recurrence of polyps
–Responded to oral steroid burst, but nasal congestion and loss of smell returned - Additional course of systemic steroids was given by family doctor
INCREASED SYSTEMIC STEROID USE
may indicate that type 2 inflammation plays a role.2
Burdensome adverse effects can be seen with
cumulative long-term use of systemic steroids.17Ben, 31steroid cycler![]() Patient profile is representative and is not an actual DUPIXENT patient.
Patient profile is representative and is not an actual DUPIXENT patient.
-
SURGERY-RELUCTANT
MEET TAYLOR
Doesn’t want surgery, seeking another option
CHOOSE DUPIXENT IF SURGERY IS OFF THE TABLE1
Taylor, 29SURGERY-RELUCTANTPatient profile is representative and is not an actual DUPIXENT patient. CLINICAL PROFILE
CLINICAL PROFILE- Diagnosed with CRSwNP and prescribed INCS
- Expresses hesitancy to undergo sinus surgery
- 2 oral steroid bursts since diagnosis but continues to experience loss of smell and nasal congestion
Most US commercial healthcare plans require documentation
of prior treatment with systemic steroids or sinus surgery.aaPatients with uncontrolled CRSwNP are eligible for DUPIXENT whether or
not they have received treatment with systemic steroids or undergone sinus surgery.1,15Taylor, 29SURGERY-RELUCTANT![]() Patient profile is representative and is not an actual DUPIXENT patient.
Patient profile is representative and is not an actual DUPIXENT patient.
-
UNCONTROLLED WITH ORAL STEROIDS
MEET DANIEL
Surgery naive and struggling with sinus symptoms
HELP UNCONTROLLED CRSwNP PATIENTS STUCK IN A CYCLE OF
SYSTEMIC STEROIDS—CHOOSE DUPIXENT1,15Daniel, 41UNCONTROLLED WITH ORAL STEROIDSPatient profile is representative and is not an actual DUPIXENT patient. CLINICAL PROFILE
CLINICAL PROFILE- History of allergic rhinitis
–Managed symptoms with decongestants, antihistamines, and INCS - Patient complained of loss of smell
- Family doctor prescribed oral steroid bursts for worsening sinus symptoms and referred to allergist for suspected CRSwNP
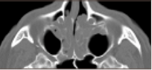
Visual examination by allergist was inconclusive, but a follow-up CT scan confirmed CRSwNP diagnosis.
Image adapted from Brescia et al. 2022.18
Type 2 inflammation often results in increased systemic steroid use.2
Burdensome adverse effects can be seen with
cumulative long-term use of systemic steroids.17![]() Daniel, 41UNCONTROLLED WITH ORAL STEROIDSPatient profile is representative and is not an actual DUPIXENT patient.
Daniel, 41UNCONTROLLED WITH ORAL STEROIDSPatient profile is representative and is not an actual DUPIXENT patient.
- History of allergic rhinitis
CRSwNP, chronic rhinosinusitis with nasal polyps; CT, computed tomography; INCS, intranasal corticosteroids; t2i, type 2 inflammation.
Frequently Asked Questions About CRSwNP
Patient Types
DUPIXENT is indicated as an add-on maintenance treatment in adult and pediatric patients aged 12 years and older with inadequately controlled chronic rhinosinusitis with nasal polyps (CRSwNP).1
Some examples of CRSwNP patients who may be appropriate for DUPIXENT are1:
- Patients who are uncontrolled despite use of systemic steroids and previous sinus surgery
- Patients who are surgery naive
- Patients with a history of asthma
- Patients with comorbid NSAID-ERD
NSAID-ERD, nonsteroidal anti-inflammatory drug–exacerbated respiratory disease.
Up to 87% of patients with CRSwNP have evidence of type 2 inflammation.11 DUPIXENT helps address the underlying type 2 inflammation in CRSwNP by targeting two of the key drivers—IL-4 and IL-13 signaling.1,12,13,*
*The mechanism of dupilumab action has not been definitively established.1
SINUS-24 and SINUS-52
DUPIXENT is appropriate for surgery-naive or -experienced patients and may provide rapid results at Day 3 for Loss of Smella and Day 2 for nasal congestion,b sustained through Week 52.1,15,16,c-f Data in adults.1,15,16
aLSM difference between DUPIXENT 300 mg Q2W + INCS (n=438) and placebo + INCS (n=286): -0.07 (95% CI: -0.12, -0.02).16,19 Patient-reported outcome. Post hoc analysis. Results are descriptive. Definitive conclusions cannot be made.16,20
bLSM difference between DUPIXENT 300 mg Q2W + INCS (n=438) and placebo + INCS (n=286): -0.07 (95% CI: -0.13, -0.01).16,19 Post hoc analysis. Results are descriptive. Definitive conclusions cannot be made.16
cLoS score at Week 24 in SINUS-52 (key secondary endpoint). LSM difference between DUPIXENT 300 mg Q2W + INCS (n=295, pooled arms) and placebo + INCS (n=153): -0.98 (95% CI: -1.15, -0.81) (P<0.0001).15
dNC score at Week 24 in SINUS-52 (coprimary endpoint). LSM difference between DUPIXENT 300 mg Q2W + INCS (n=295, pooled arms) and placebo + INCS (n=153): -0.87 (95% CI: -1.03, -0.71) (P<0.0001).1,15
eLoS score at Week 52 in SINUS-52. LSM difference between DUPIXENT 300 mg Q2W + INCS (n=150) and placebo + INCS (n=153): -1.10 (95% CI: -1.31, -0.89).1 Analysis was not multiplicity controlled. Results are descriptive.15
fNC score at Week 52 in SINUS-52 (key secondary endpoint). LSM difference between DUPIXENT 300 mg Q2W + INCS (n=150) and placebo + INCS (n=153): -0.98 (95% CI: -1.17, -0.79) (P<0.0001).1,15
Loss of Smell (LoS) score (range 0-3): reduced score indicates improvement.1
Nasal congestion/obstruction (NC) score (range 0-3): reduced score indicates improvement.1
CRSwNP, chronic rhinosinusitis with nasal polyps; INCS, intranasal corticosteroids; LSM, least squares mean; Q2W, once every 2 weeks.
Use of DUPIXENT for the CRSwNP indication in patients aged 12-17 years is supported by evidence from studies of DUPIXENT as add-on maintenance treatment in adults with inadequately controlled CRSwNP.1
)



