Train patients and/or caregivers on proper administration prior to use1
- In adults with bullous pemphigoid, use DUPIXENT in combination with a tapering course of oral corticosteroids
- Once disease control has occurred, gradually taper corticosteroids, after which continue DUPIXENT as monotherapy1
- In case of relapse, corticosteroids may be added if medically advisable
IN ADULTS
18+
YEARS
One dosage regimen
regardless of weight
Initial loading dose:
600 mg
2 x 300 mg
Followed by:
300 mg Q2W
1 x 300 mg
Get the full
dosing regimen
Email Dosing Regimen
For supplemental injection training, visit our Patient Video Injection Support page
Q2W, once every 2 weeks.
Consider before injecting1
- DUPIXENT is an injectable medicine that is administered by subcutaneous injection and is intended for use under the
guidance of a healthcare provider - Provide proper training to patients and/or caregivers on the preparation and administration of DUPIXENT prior to use,
according to the Instructions for Use - Rotate injection site with each injection
- Consider completing all age-appropriate vaccinations as recommended by current immunization guidelines prior to
initiating treatment with DUPIXENT
At-home or in-office administration options
Important administration
instructions1,2
Preparation and administration of DUPIXENT
Preparation and administration of DUPIXENT
- Patients and/or caregivers should read the Instructions for Use prior to injecting
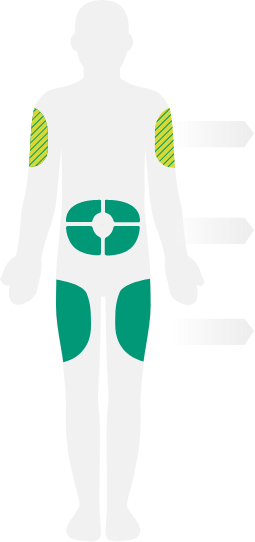
- Instruct patients and/or caregivers to administer subcutaneous
injection into the thigh or abdomen, except for the 2 inches (5 cm)
around the navel - The upper arm can also be used if a caregiver administers the injection
- It is important to rotate the injection site with each injection. DO NOT inject DUPIXENT into skin that is tender, damaged, bruised, or scarred
- For the initial dose, patients and/or caregivers should administer each DUPIXENT injection at a different injection site
See the Instructions for Use for more detailed instructions on the preparation and administration of DUPIXENT.
For supplemental injection training, visit our
Patient Video Injection Support page
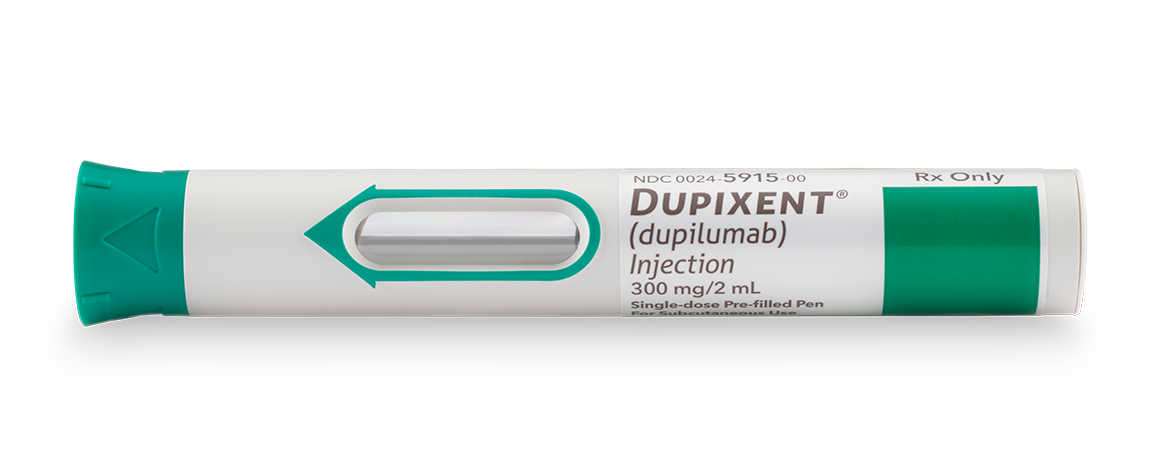
Pre-filled Pen
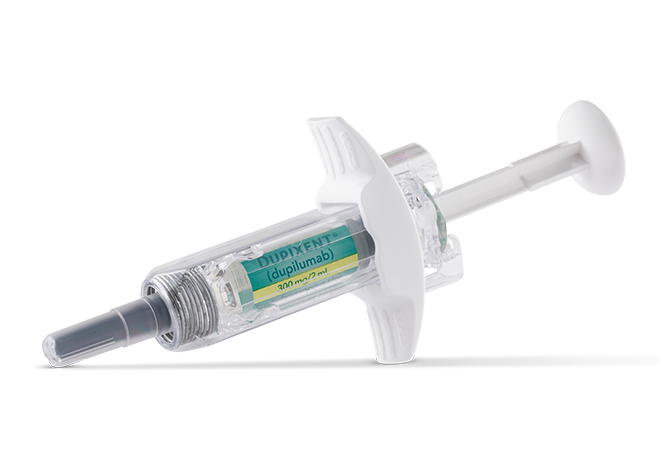
Pre-filled Syringe
ADDITIONAL DOSAGE INFORMATION
Before injection, instruct patients and/or caregivers to remove the DUPIXENT pre‑filled pen or syringe from the refrigerator and allow DUPIXENT to reach room temperature without removing the needle cap.
- 45 minutes for the 300 mg/2 mL pre-filled pen or syringe
Patients and/or caregivers should not use the pre-filled pen or syringe if the liquid contains visible particulate matter, or is discolored or cloudy (other than clear to slightly opalescent, colorless to pale yellow).
Patients and/or caregivers should discard any unused product remaining in the pre-filled pen or syringe in accordance with local requirements.
For supplemental injection training, visit our
Patient Video Injection Support Page
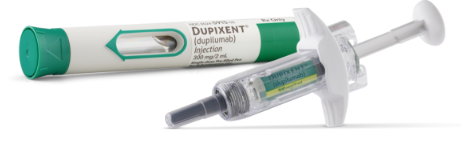
DUPIXENT is a clear to slightly opalescent, colorless to pale yellow solution available as:
- Injection: 300 mg/2 mL in a single-dose, pre-filled pen with hidden needle or syringe with needle shield
DUPIXENT is available in cartons containing 2 pre-filled pens or syringes with needle shields. Each 300 mg pre-filled syringe or pre-filled pen delivers 300 mg/2 mL.
- DUPIXENT is sterile and preservative-free. Patients and/or caregivers should discard any unused portion
- Patients and/or caregivers should store DUPIXENT by refrigerating at 36 °F to 46 °F (2 °C to 8 °C) in the original carton to protect from light
- If necessary, pre-filled pens or syringes may be kept at room temperature up to 77 °F (25 °C) for a maximum of 14 days. DUPIXENT should not be stored above 77 °F (25 °C). After removal from the refrigerator, DUPIXENT must be used within 14 days or discarded
- The pen or syringe should not be exposed to heat or direct sunlight
- Any unused medicinal product or waste material should be disposed of in accordance with local requirements
- Do NOT freeze. Do NOT expose to heat. Do NOT shake
DUPIXENT MyWay®